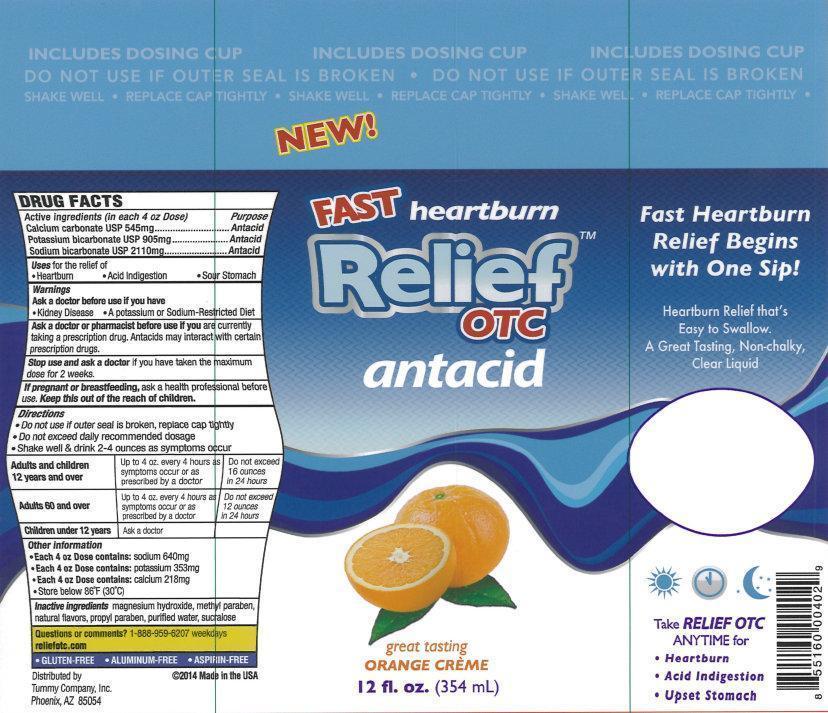
Label: RELIEF OTC ANTACID- calcium carbonate, potassium bicarbonate, sodium bicarbonate liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 69016-001-18, 69016-001-54 - Packager: Tummy Company, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated May 29, 2014
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients (in each 4 oz Dose)
- Purpose
- Uses
- Warnings
-
Directions
- Do not use if outer seal is broken, replace cap tightly
- Do not exceed daily recommended dosage
- Shake well & drink 2-4 ounces as symptoms occur
Adults and children 12 years and over Up to 4oz. every 4 hours as sumptoms occur or as prescribed by a doctor Do not exceed 16 ounces in 24 hours Adults 60 and over Up to 4oz. every 4 hours as sumptoms occur or as prescribed by a doctor Do not exceed 12 ounces in 24 hours Children under 12 years Ask a doctor - Other information
- Inactive ingredients
- Questions or comments?
-
Representative Label
New!
Fast heartburn Relief
OTC
antacid
great tasting
ORANGE CREME
12 fl. oz. (354 mL)
Fast Heartburn Relief Begins with One Sip!
Heartburn Relief that's Easy to Swallow.
A Great Tasting, Non-Chalky, Clear Liquid
Take RELIEF OTC ANYTIME for
- Hearburn
- Acid Indigestion
- Upset Stomach
- GLUTEN-FREE
- ALUMINUM-FREE
- ASPIRIN-FREE
Distributed by
Tummy Company, Inc.
Phoenix,
AZ 85054
2014 Made in the USA
-
INGREDIENTS AND APPEARANCE
RELIEF OTC ANTACID
calcium carbonate, potassium bicarbonate, sodium bicarbonate liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69016-001 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CALCIUM CARBONATE (UNII: H0G9379FGK) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM CATION 545 mg in 118 mL POTASSIUM BICARBONATE (UNII: HM5Z15LEBN) (POTASSIUM CATION - UNII:295O53K152) POTASSIUM CATION 905 mg in 118 mL SODIUM BICARBONATE (UNII: 8MDF5V39QO) (SODIUM CATION - UNII:LYR4M0NH37) SODIUM BICARBONATE 2110 mg in 118 mL Inactive Ingredients Ingredient Name Strength MAGNESIUM HYDROXIDE (UNII: NBZ3QY004S) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) SUCRALOSE (UNII: 96K6UQ3ZD4) Product Characteristics Color Score Shape Size Flavor ORANGE (Orange Creme) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69016-001-18 118 mL in 1 BOTTLE 2 NDC:69016-001-54 354 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part331 05/28/2014 Labeler - Tummy Company, Inc. (079165926) Establishment Name Address ID/FEI Business Operations Alera Technologies Inc. 962055740 manufacture(69016-001)