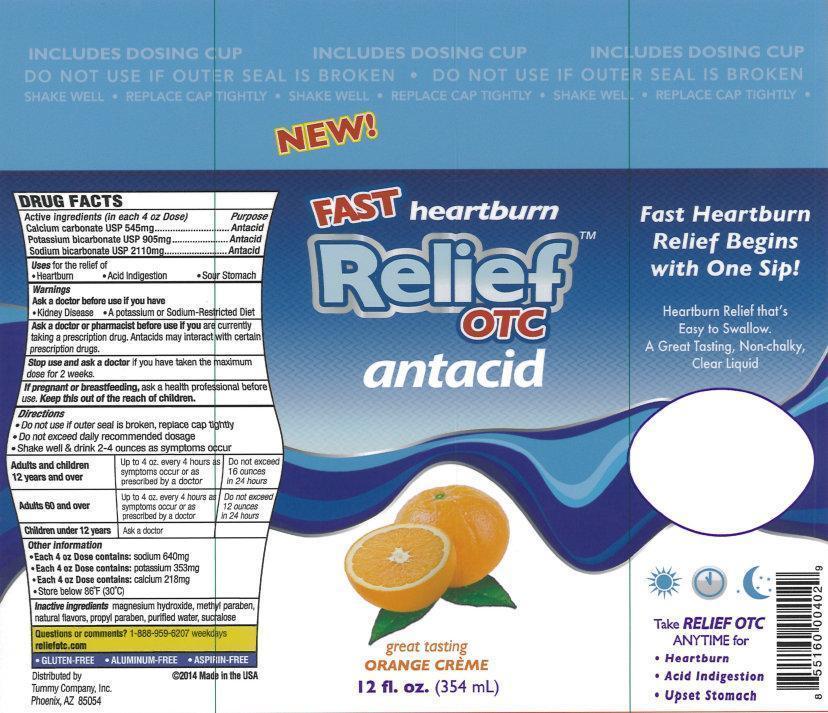
Active ingredients (in each 4 oz Dose)
Calcium carbonate USP 545mg
Potassium bicarbonate USP 905mg
Sodium bicarbonate USP 2110mg
Warnings
Directions
- Do not use if outer seal is broken, replace cap tightly
- Do not exceed daily recommended dosage
- Shake well & drink 2-4 ounces as symptoms occur
| Adults and children 12 years and over | Up to 4oz. every 4 hours as sumptoms occur or as prescribed by a doctor | Do not exceed 16 ounces in 24 hours |
| Adults 60 and over | Up to 4oz. every 4 hours as sumptoms occur or as prescribed by a doctor | Do not exceed 12 ounces in 24 hours |
| Children under 12 years | Ask a doctor |
Other information
- Each 4 oz Dose contains: sodium 640mg
- Each 4 oz Dose contains: potassium 353mg
- Each 4 oz Dose contains: calcium 218mg
- Store below 86 degrees F (30 degrees C)
Inactive ingredients
magnesium hydroxide, metyl paraben, natural flavors, propyl paraben, purified water, sucralose
Representative Label
New!
Fast heartburn Relief
OTC
antacid
great tasting
ORANGE CREME
12 fl. oz. (354 mL)
Fast Heartburn Relief Begins with One Sip!
Heartburn Relief that's Easy to Swallow.
A Great Tasting, Non-Chalky, Clear Liquid
Take RELIEF OTC ANYTIME for
- Hearburn
- Acid Indigestion
- Upset Stomach
- GLUTEN-FREE
- ALUMINUM-FREE
- ASPIRIN-FREE
Distributed by
Tummy Company, Inc.
Phoenix,
AZ 85054
2014 Made in the USA