Label: EUCERIN ITCH RELIEF INTENSIVE CALMING- menthol lotion
- NDC Code(s): 10356-358-29, 10356-358-51
- Packager: Beiersdorf Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 29, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
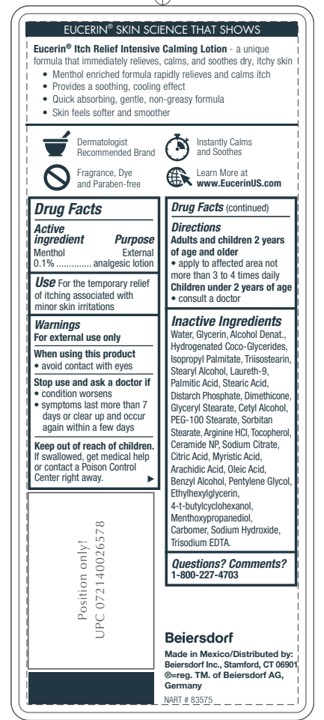
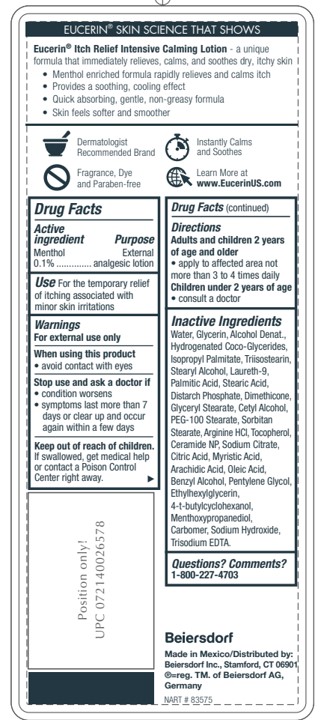
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- QUESTIONS
-
INACTIVE INGREDIENT
Ingredients:
Water, Glycerin, Alcohol Denat., Hydrogenated Coco-Glycerides, Isopropyl Palmitate, Triisostearin,
Stearyl Alcohol, Laureth-9, Palmitic Acid, Stearic Acid, Distarch Phosphate, Dimethicone,
Glyceryl Stearate, Cetyl Alcohol, PEG-100 Stearate, Sorbitan Stearate, Arginine HCl, Tocopherol,
Ceramide NP, Sodium Citrate, Citric Acid, Myristic Acid, Arachidic Acid, Oleic Acid,
Benzyl Alcohol, Pentylene Glycol, Ethylhexylglycerin, 4-t-butylcyclohexanol,
Menthoxypropanediol, Carbomer, Sodium Hydroxide, Trisodium EDTA.Inactive Ingredients
- DOSAGE & ADMINISTRATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
EUCERIN ITCH RELIEF INTENSIVE CALMING
menthol lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10356-358 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 0.1 g in 100 mL Inactive Ingredients Ingredient Name Strength CETYL ALCOHOL (UNII: 936JST6JCN) DIMETHICONE (UNII: 92RU3N3Y1O) SODIUM CITRATE (UNII: 1Q73Q2JULR) BENZYL ALCOHOL (UNII: LKG8494WBH) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) ALCOHOL (UNII: 3K9958V90M) PEG-100 STEARATE (UNII: YD01N1999R) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) CARBOMER 980 (UNII: 4Q93RCW27E) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SORBITAN MONOSTEARATE (UNII: NVZ4I0H58X) ARGININE HYDROCHLORIDE (UNII: F7LTH1E20Y) PENTYLENE GLYCOL (UNII: 50C1307PZG) 4-TERT-BUTYLCYCLOHEXANOL (UNII: K0H1405S9C) 3-((L-MENTHYL)OXY)PROPANE-1,2-DIOL (UNII: KD6TZ2QICH) SODIUM HYDROXIDE (UNII: 55X04QC32I) EDETATE TRISODIUM (UNII: 420IP921MB) TOCOPHEROL (UNII: R0ZB2556P8) CERAMIDE NP (UNII: 4370DF050B) MYRISTIC ACID (UNII: 0I3V7S25AW) ARACHIDIC ACID (UNII: PQB8MJD4RB) OLEIC ACID (UNII: 2UMI9U37CP) TRIISOSTEARIN (UNII: 71503RH8KG) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) PALMITIC ACID (UNII: 2V16EO95H1) STEARIC ACID (UNII: 4ELV7Z65AP) HYDROGENATED COCO-GLYCERIDES (UNII: XDD37N2GPR) ISOPROPYL PALMITATE (UNII: 8CRQ2TH63M) POLIDOCANOL (UNII: 0AWH8BFG9A) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10356-358-51 250 mL in 1 BOTTLE; Type 0: Not a Combination Product 06/15/2020 2 NDC:10356-358-29 14 mL in 1 BOTTLE; Type 0: Not a Combination Product 06/15/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 06/15/2020 Labeler - Beiersdorf Inc (001177906)