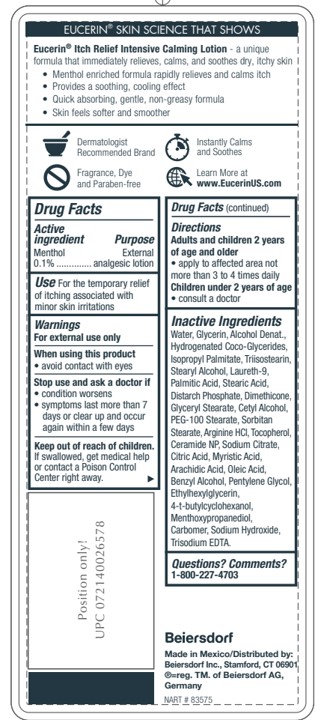
Stop use and ask a doctor if
- condition worsens
- symptoms last more than 7 days or clear up and occur again within a few days
- irritation occurs
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Ingredients:
Water, Glycerin, Alcohol Denat., Hydrogenated Coco-Glycerides, Isopropyl Palmitate, Triisostearin,
Stearyl Alcohol, Laureth-9, Palmitic Acid, Stearic Acid, Distarch Phosphate, Dimethicone,
Glyceryl Stearate, Cetyl Alcohol, PEG-100 Stearate, Sorbitan Stearate, Arginine HCl, Tocopherol,
Ceramide NP, Sodium Citrate, Citric Acid, Myristic Acid, Arachidic Acid, Oleic Acid,
Benzyl Alcohol, Pentylene Glycol, Ethylhexylglycerin, 4-t-butylcyclohexanol,
Menthoxypropanediol, Carbomer, Sodium Hydroxide, Trisodium EDTA.Inactive Ingredients