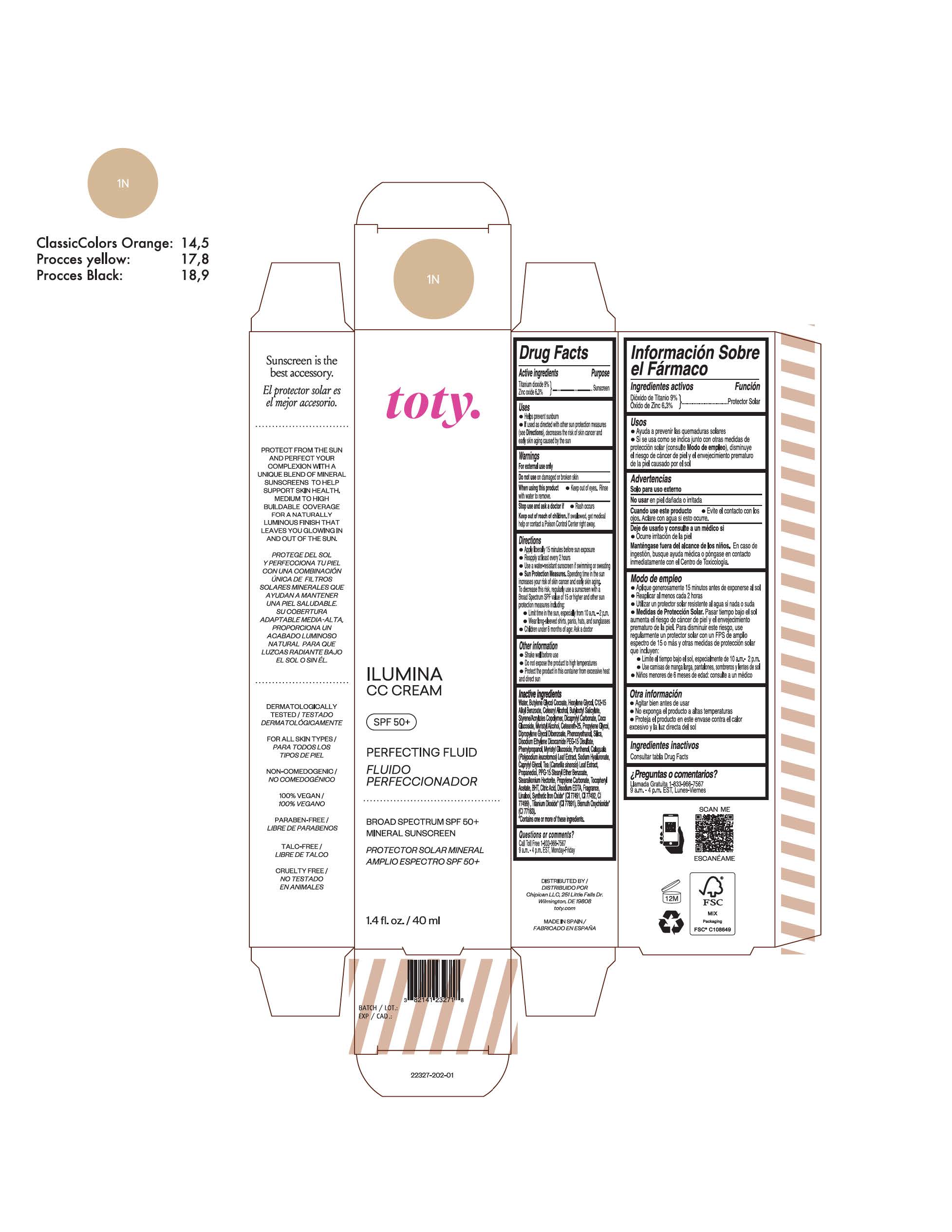
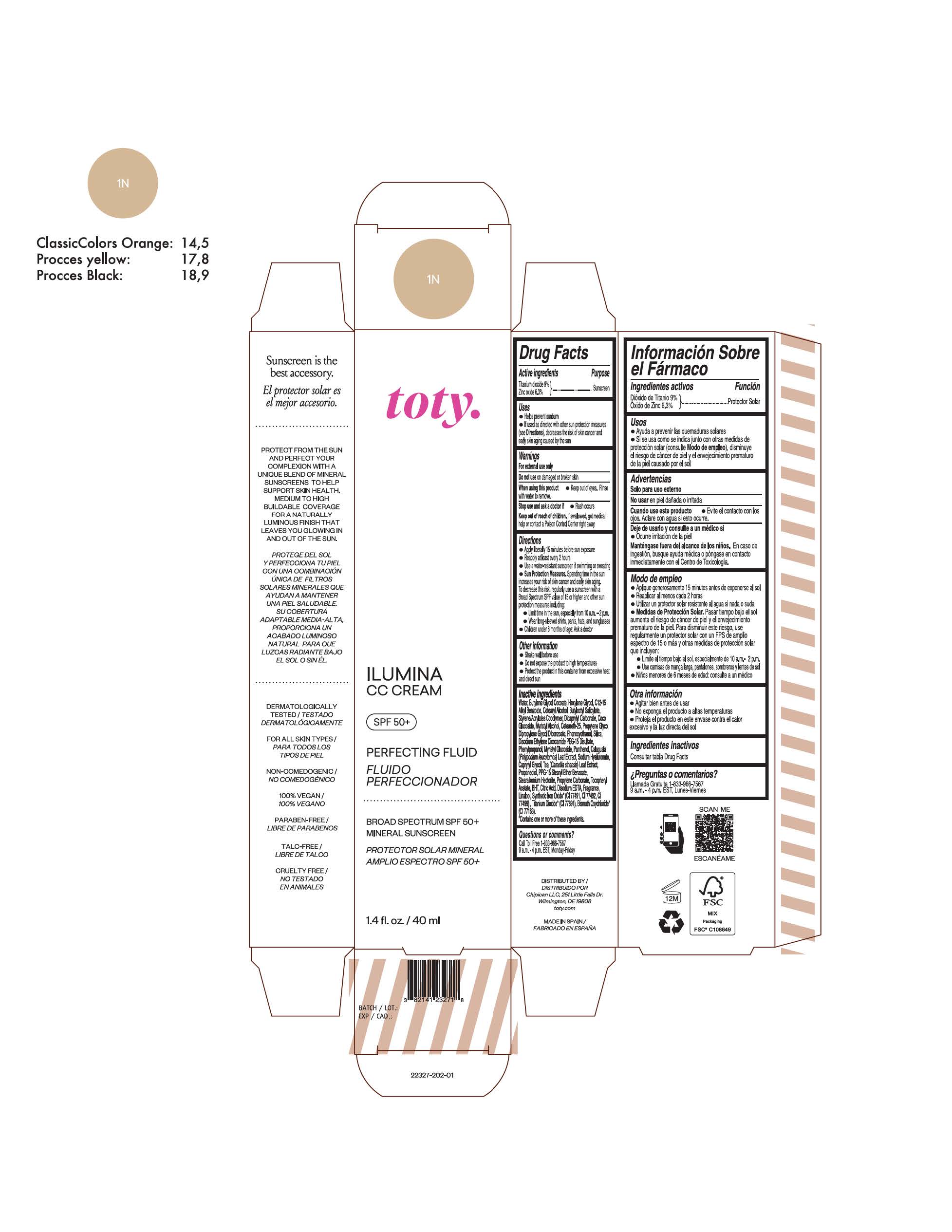
Label: TOTY ILUMINA CC CREAM 1N- titanium dioxide, zinc oxide cream
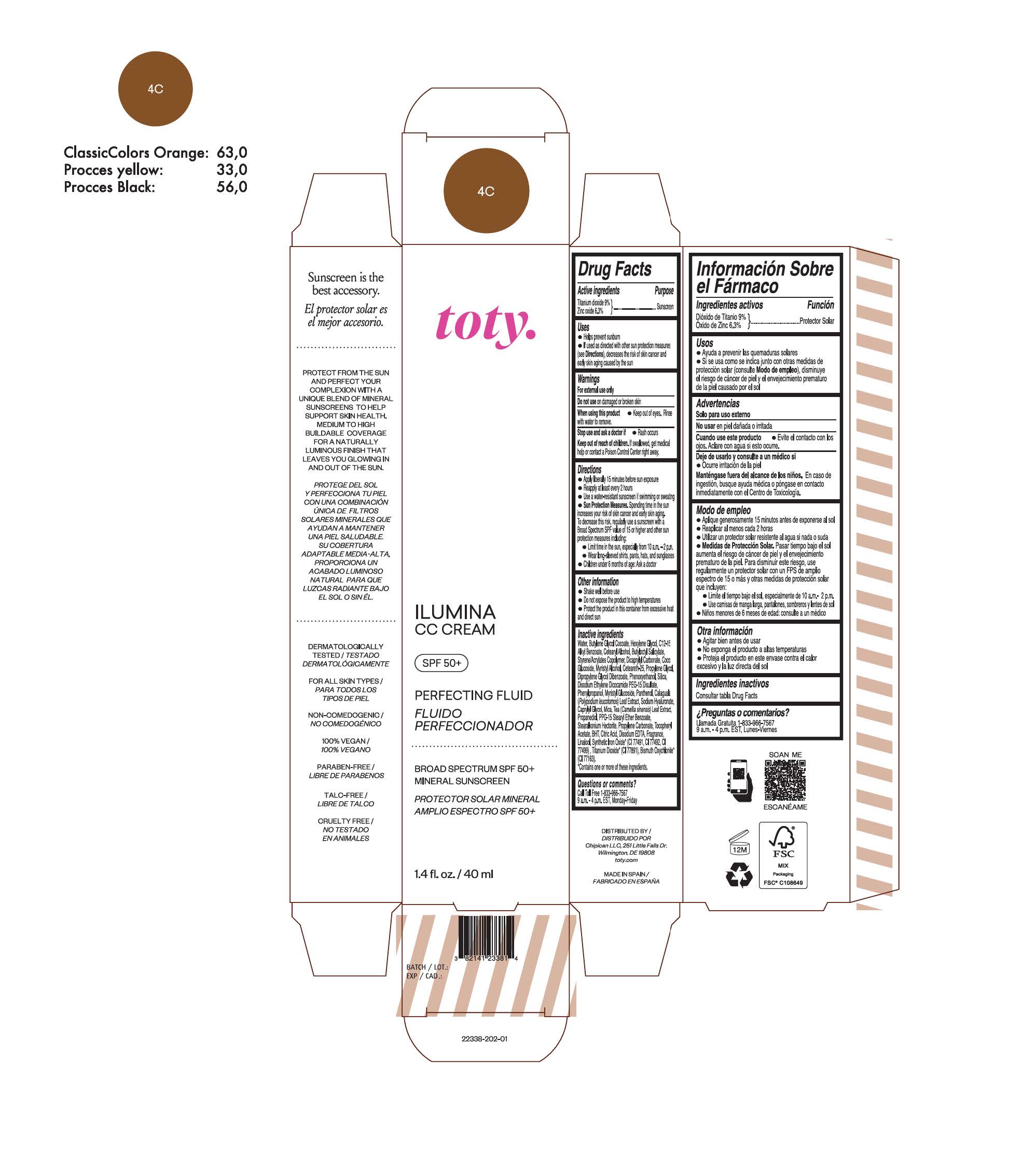
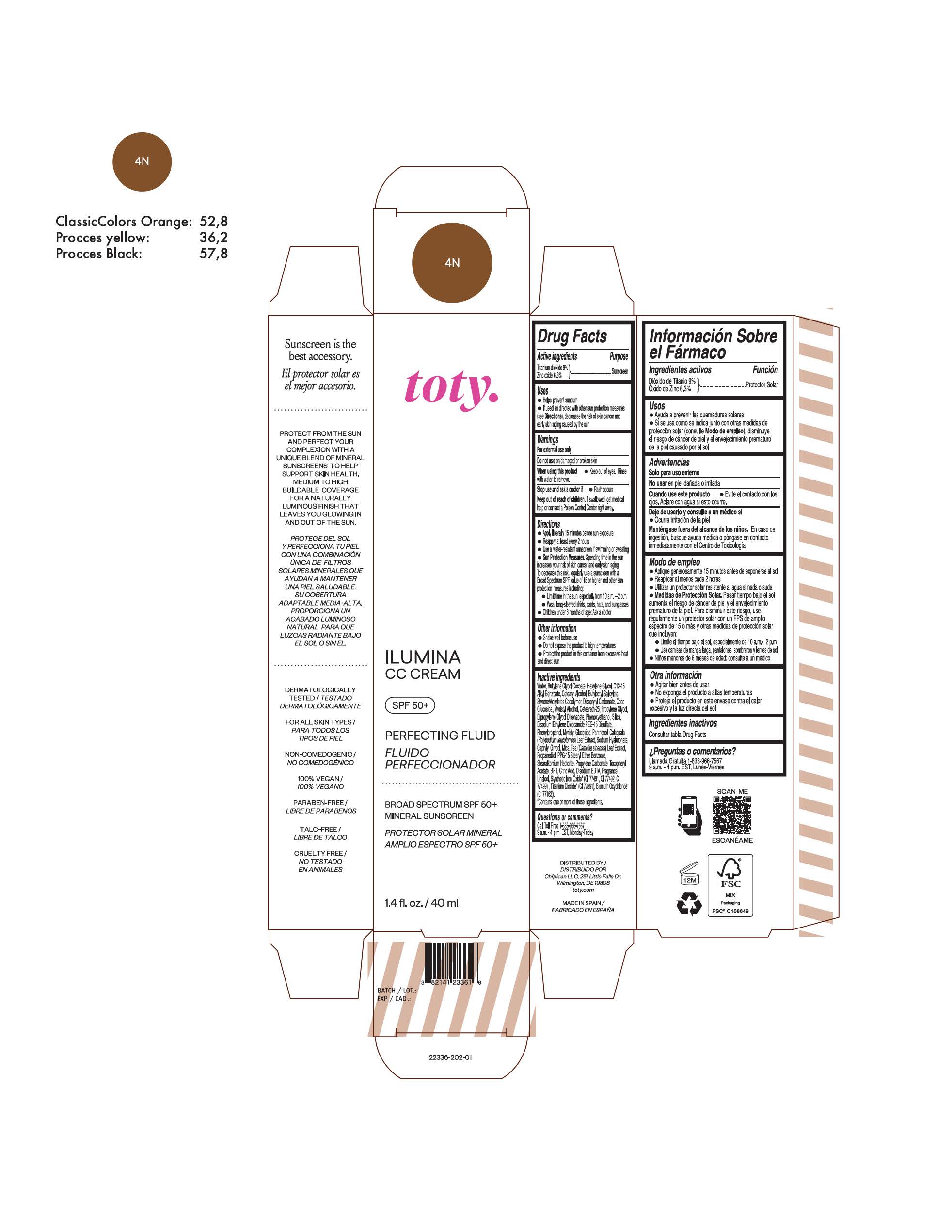
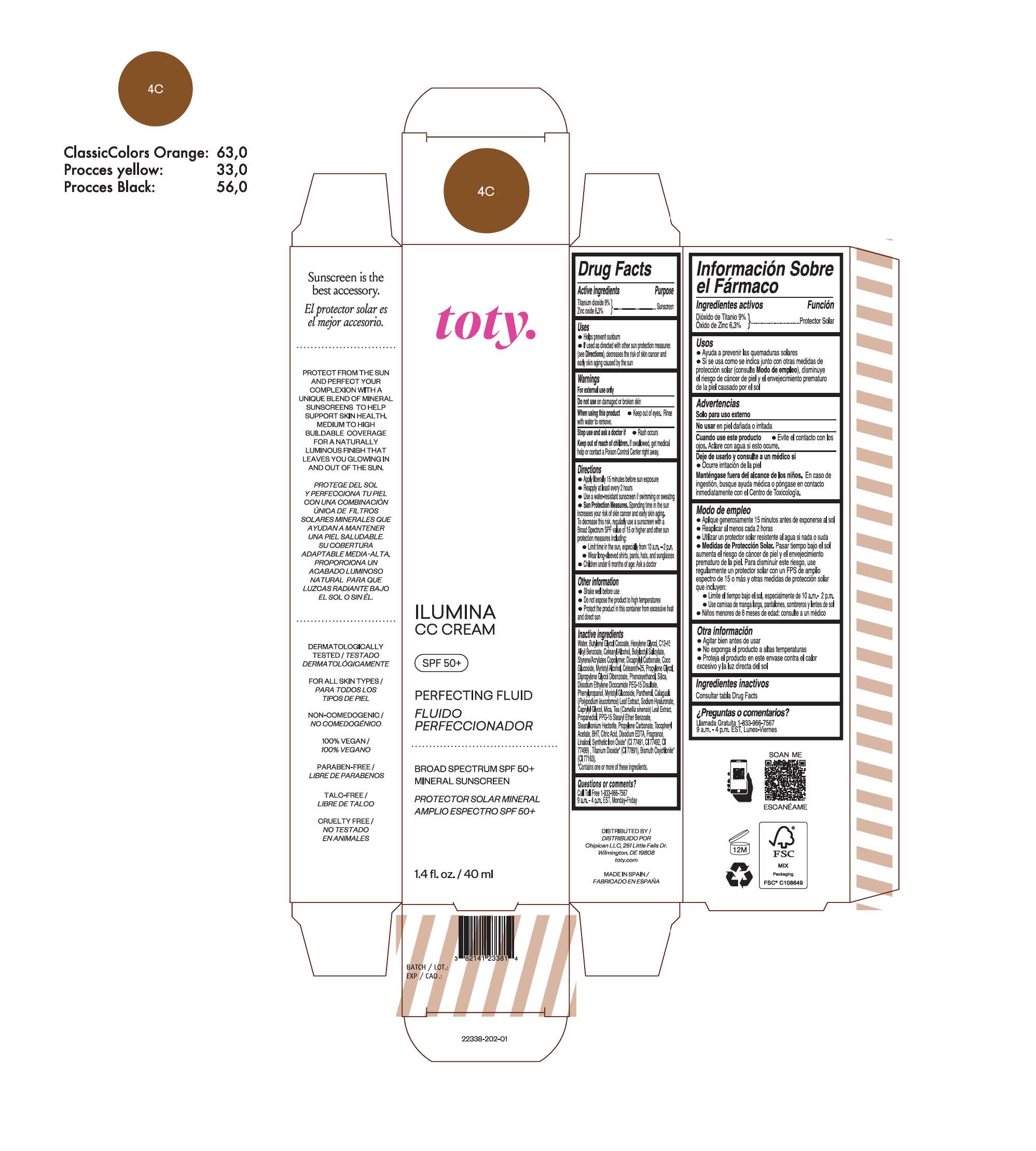
TOTY ILUMINA CC CREAM 4C- titanium dioxide, zinc oxide cream
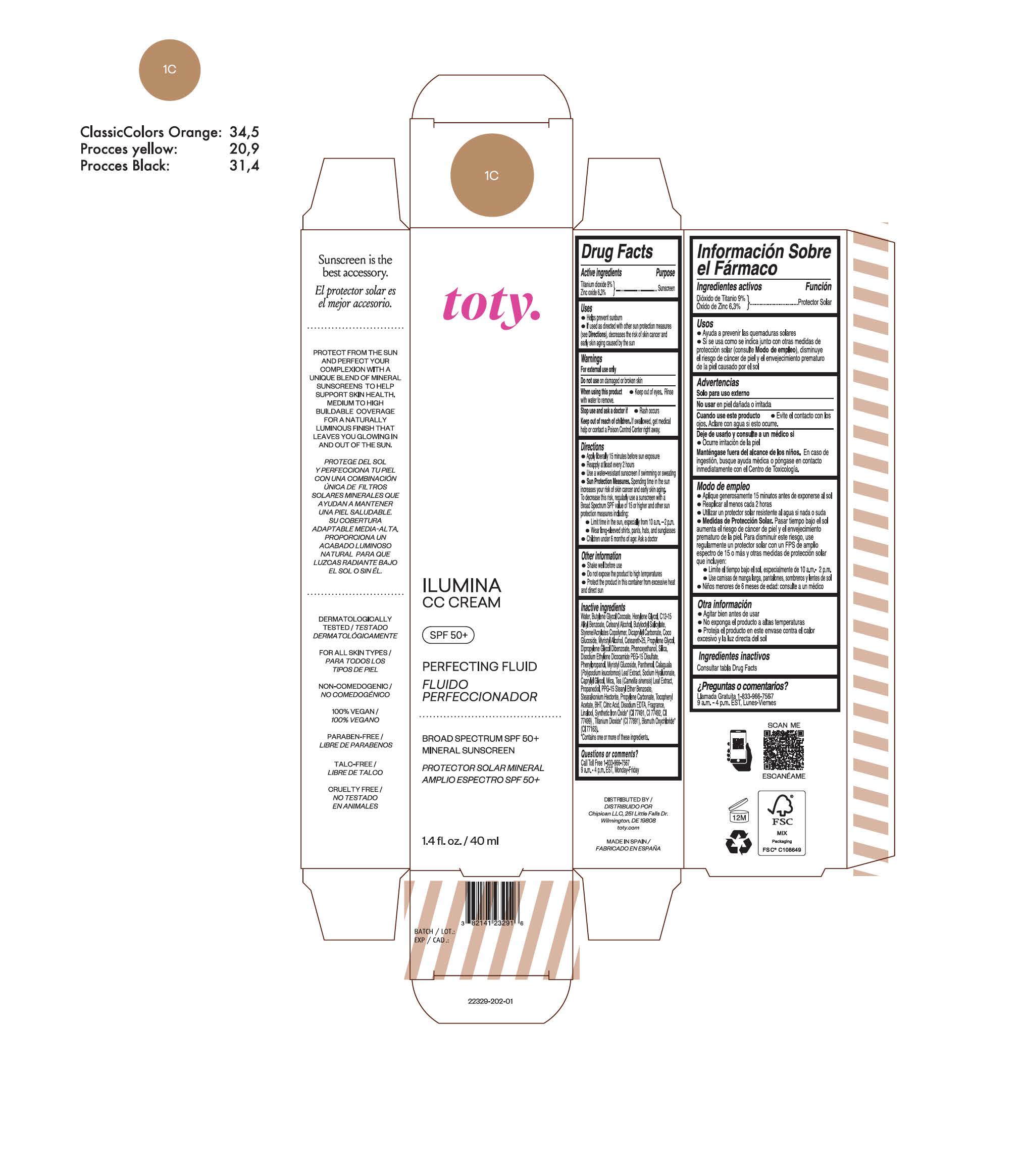
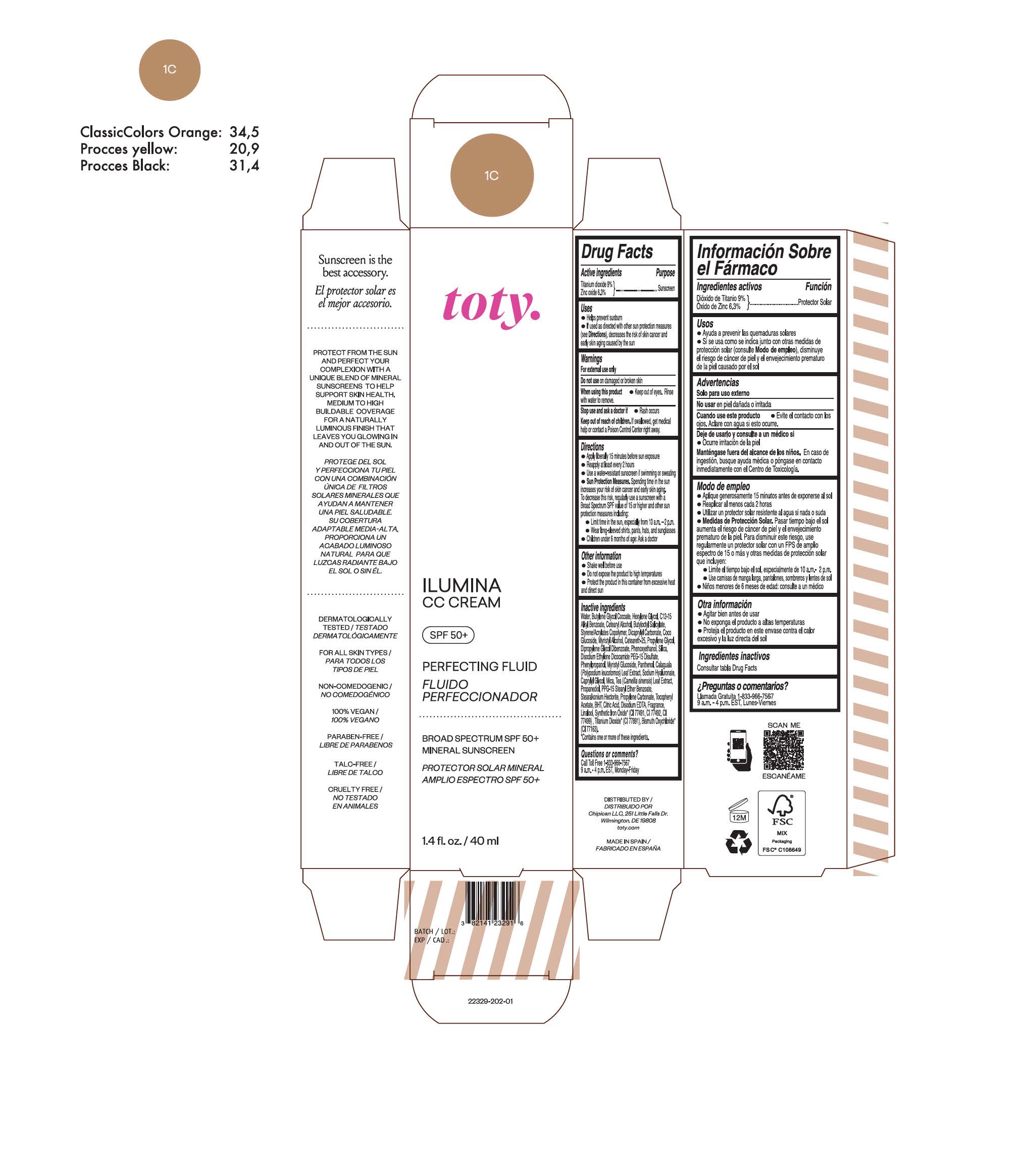
TOTY ILUMINA CC CREAM 1C- titanium dioxide, zinc oxide cream
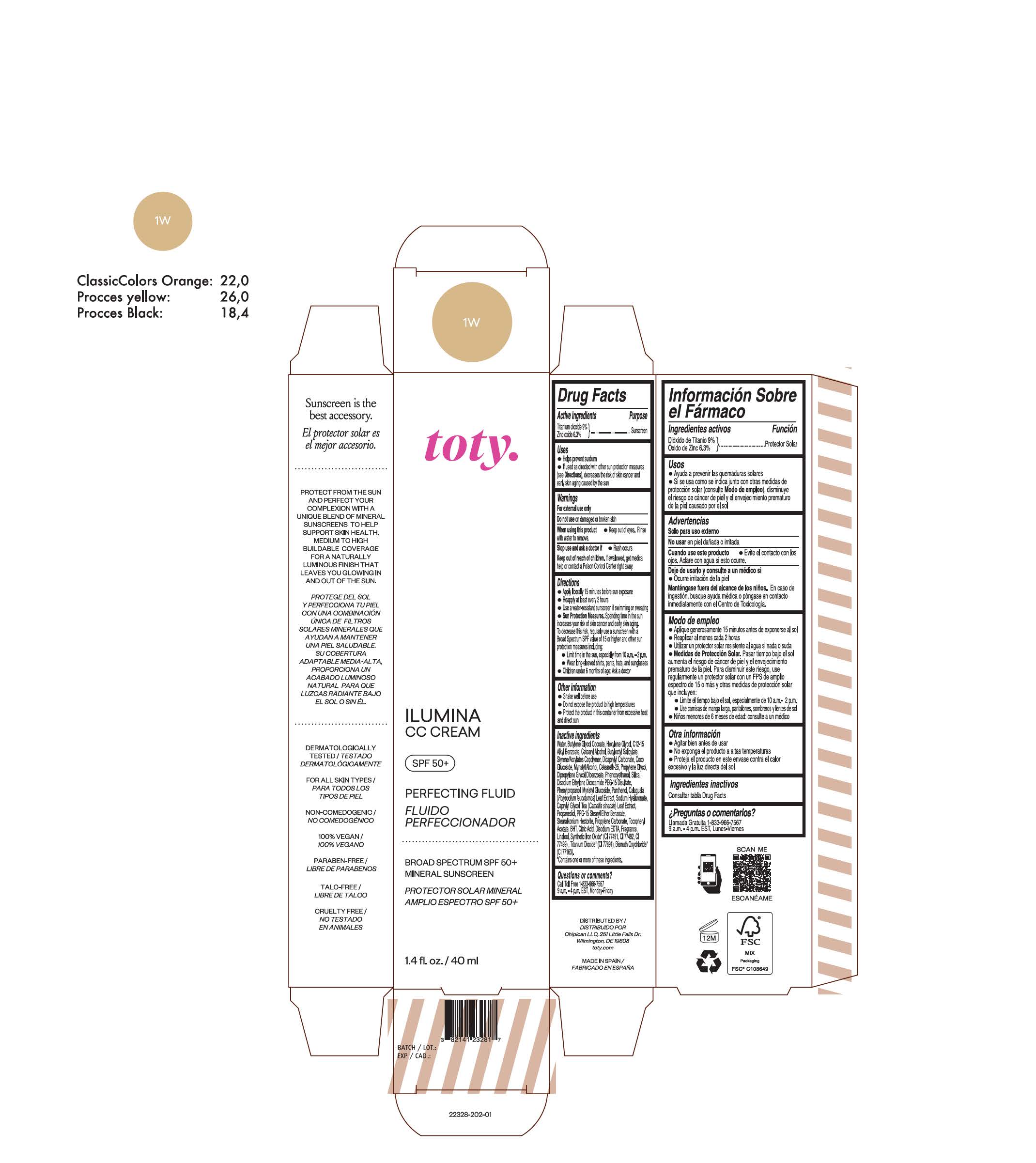
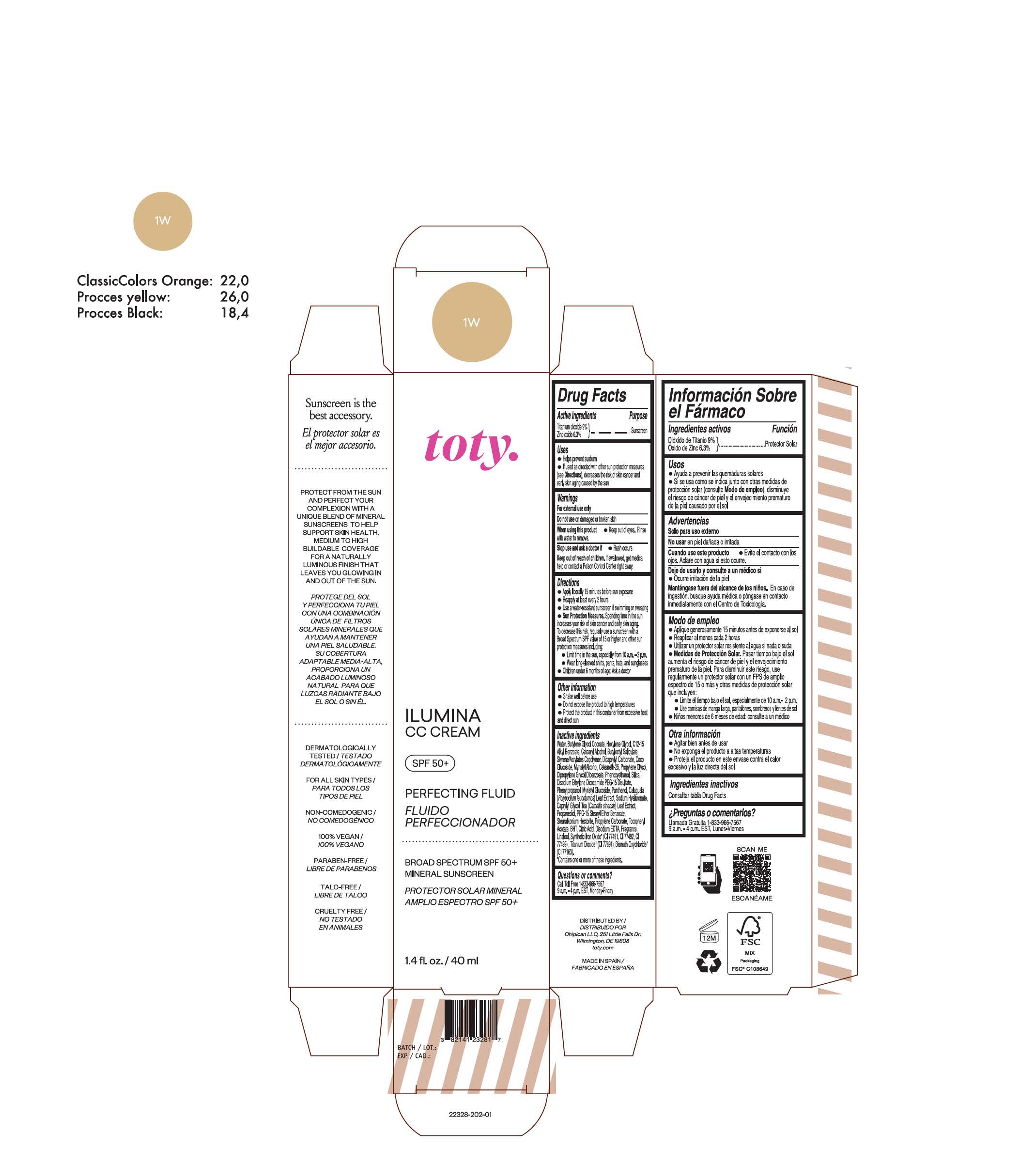
TOTY ILUMINA CC CREAM 1W- titanium dioxide, zinc oxide cream
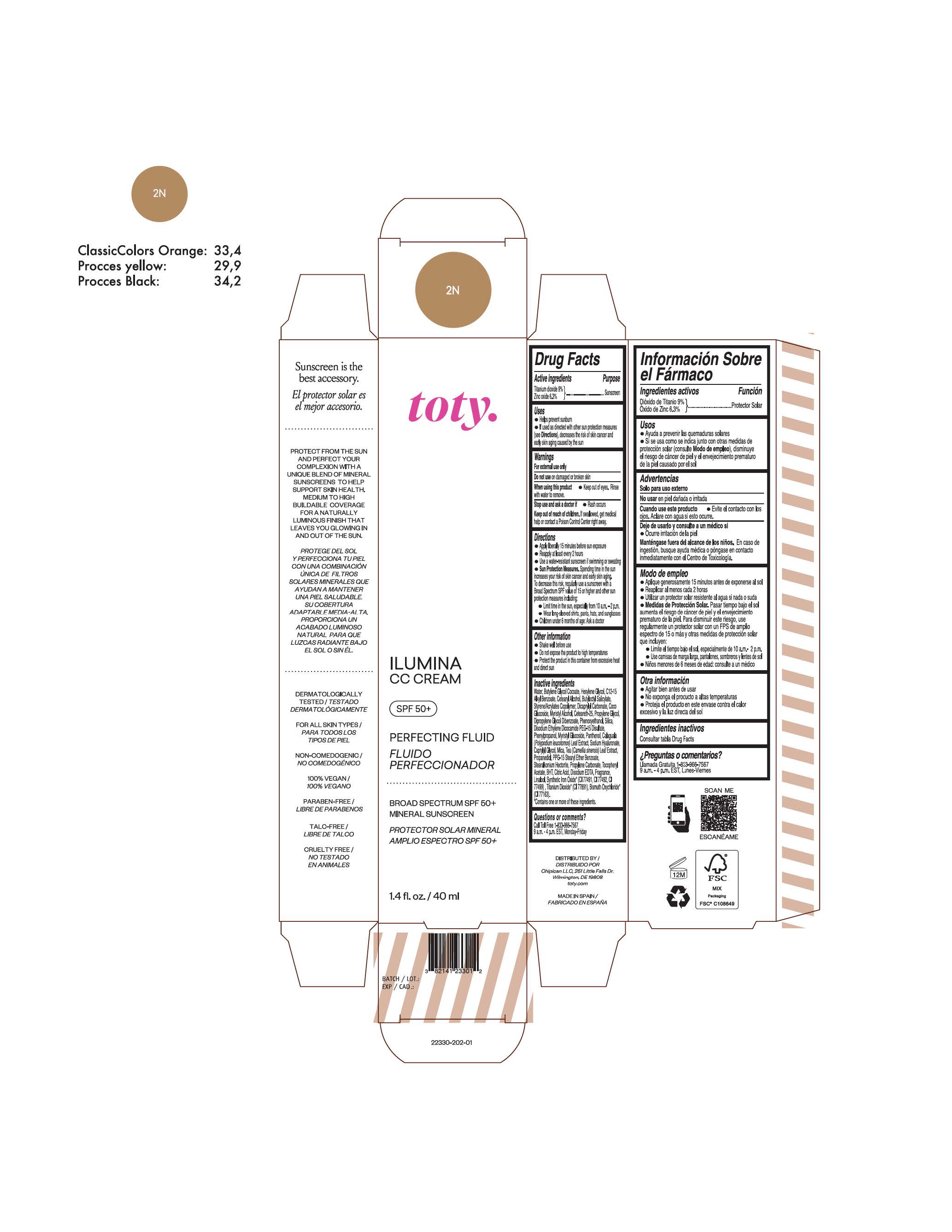
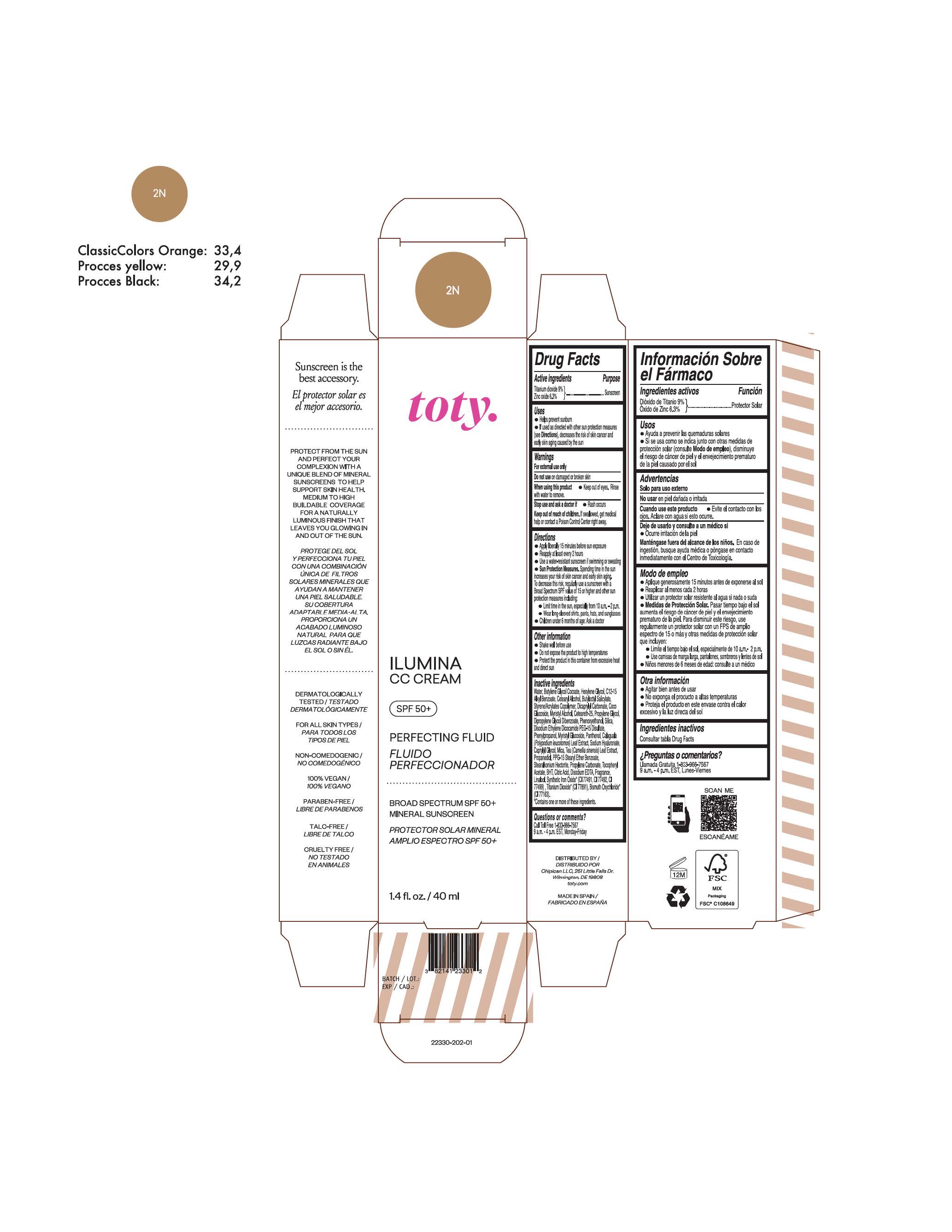
TOTY ILUMINA CC CREAM 2N- titanium dioxide, zinc oxide cream
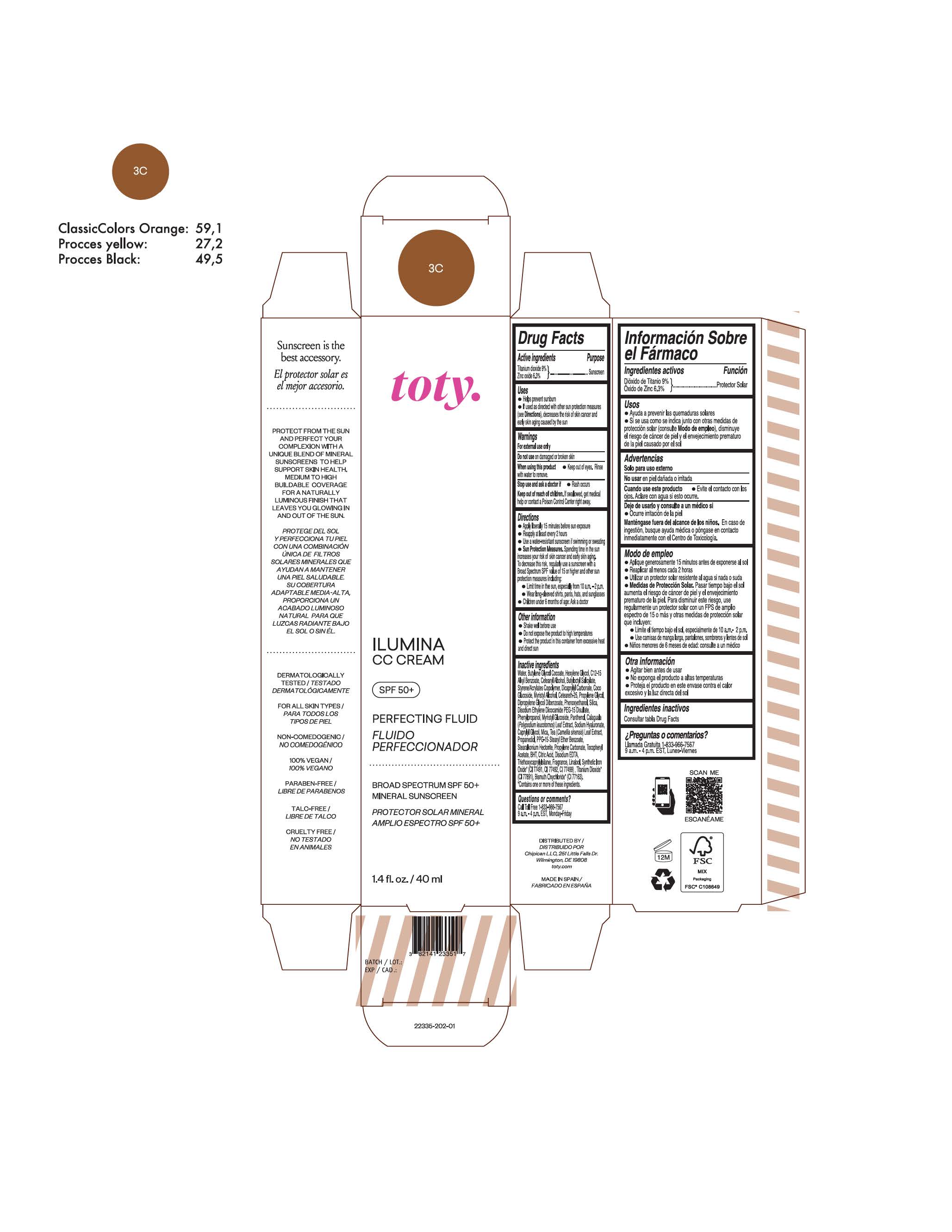
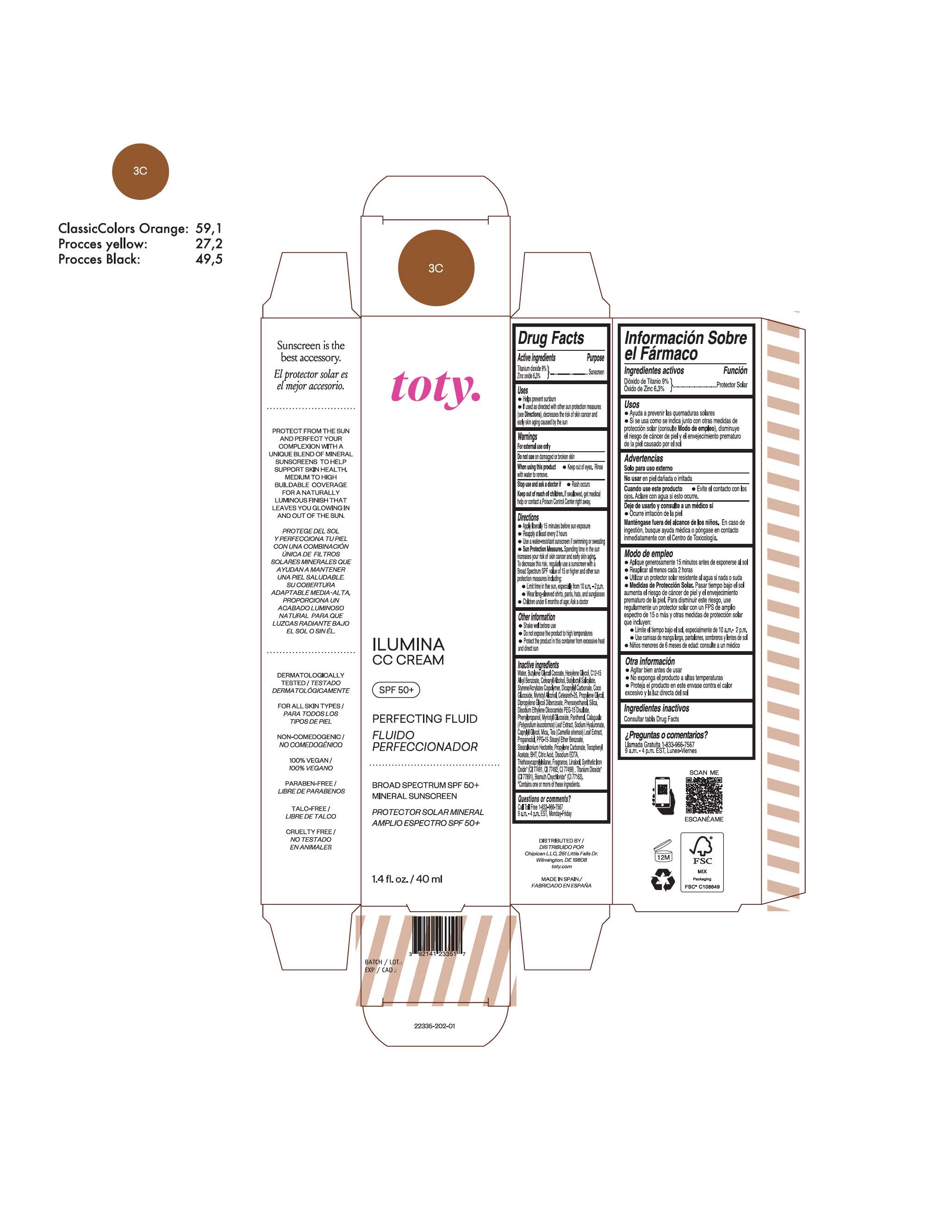
TOTY ILUMINA CC CREAM 3C- titanium dioxide, zinc oxide cream
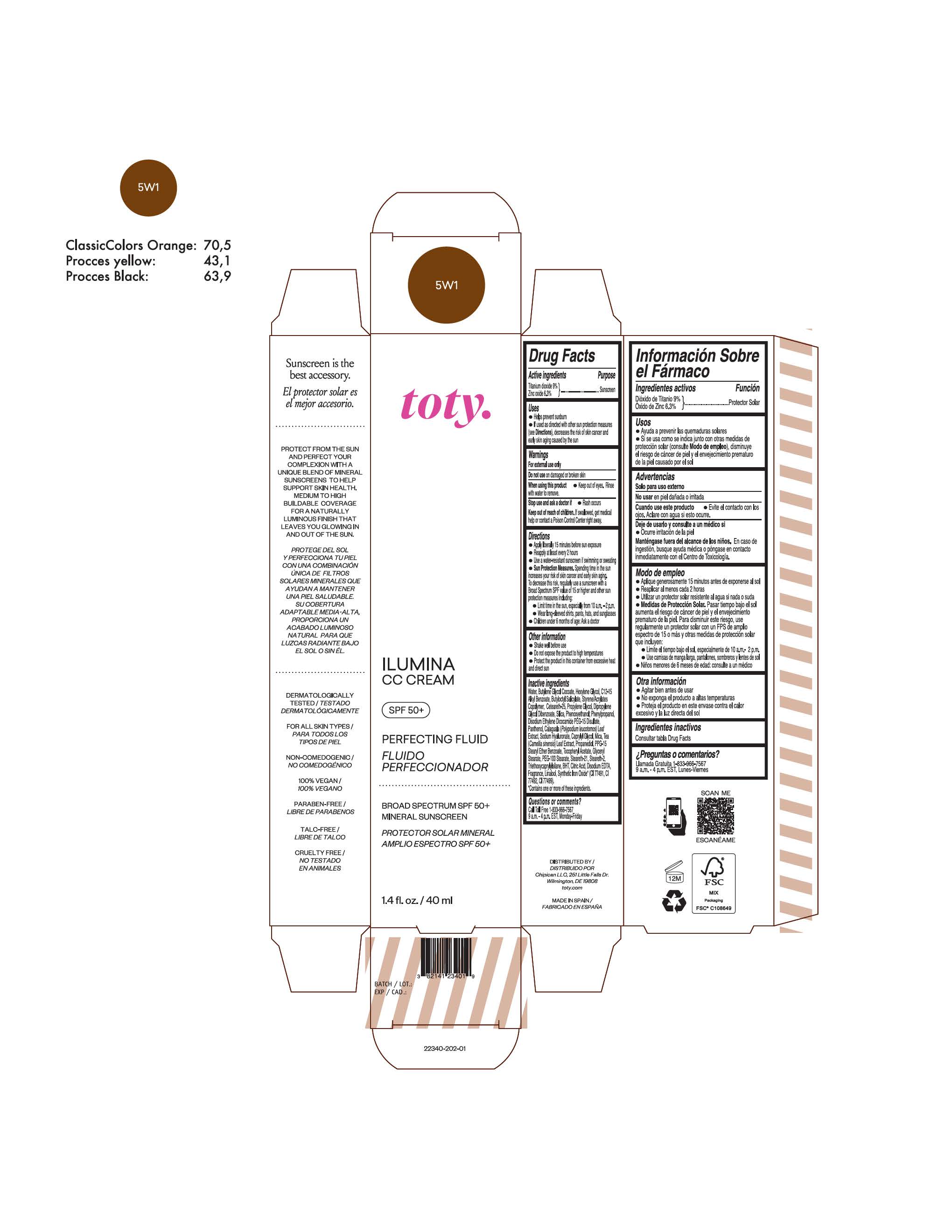
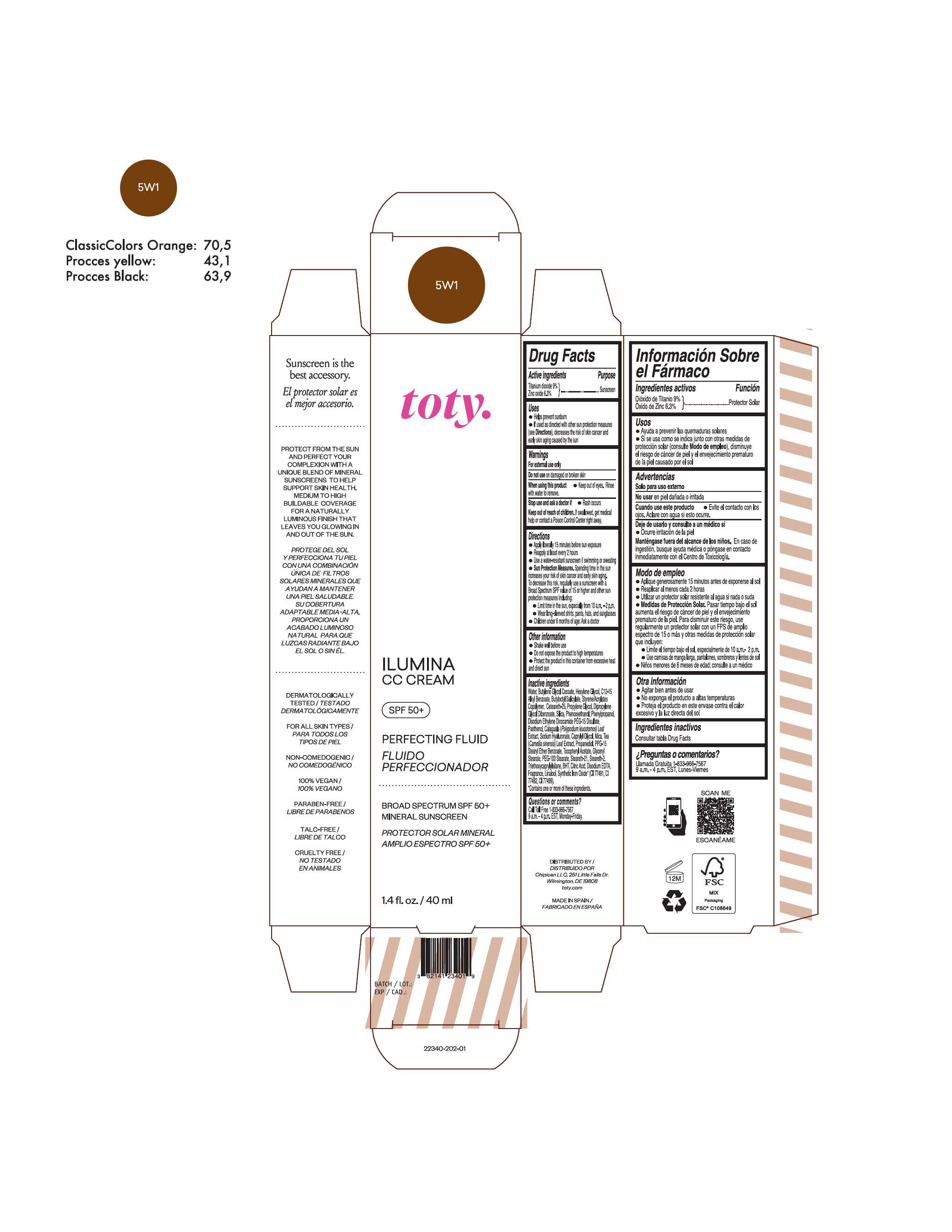
TOTY ILUMINA CC CREAM 5W1- titanium dioxide, zinc oxide cream
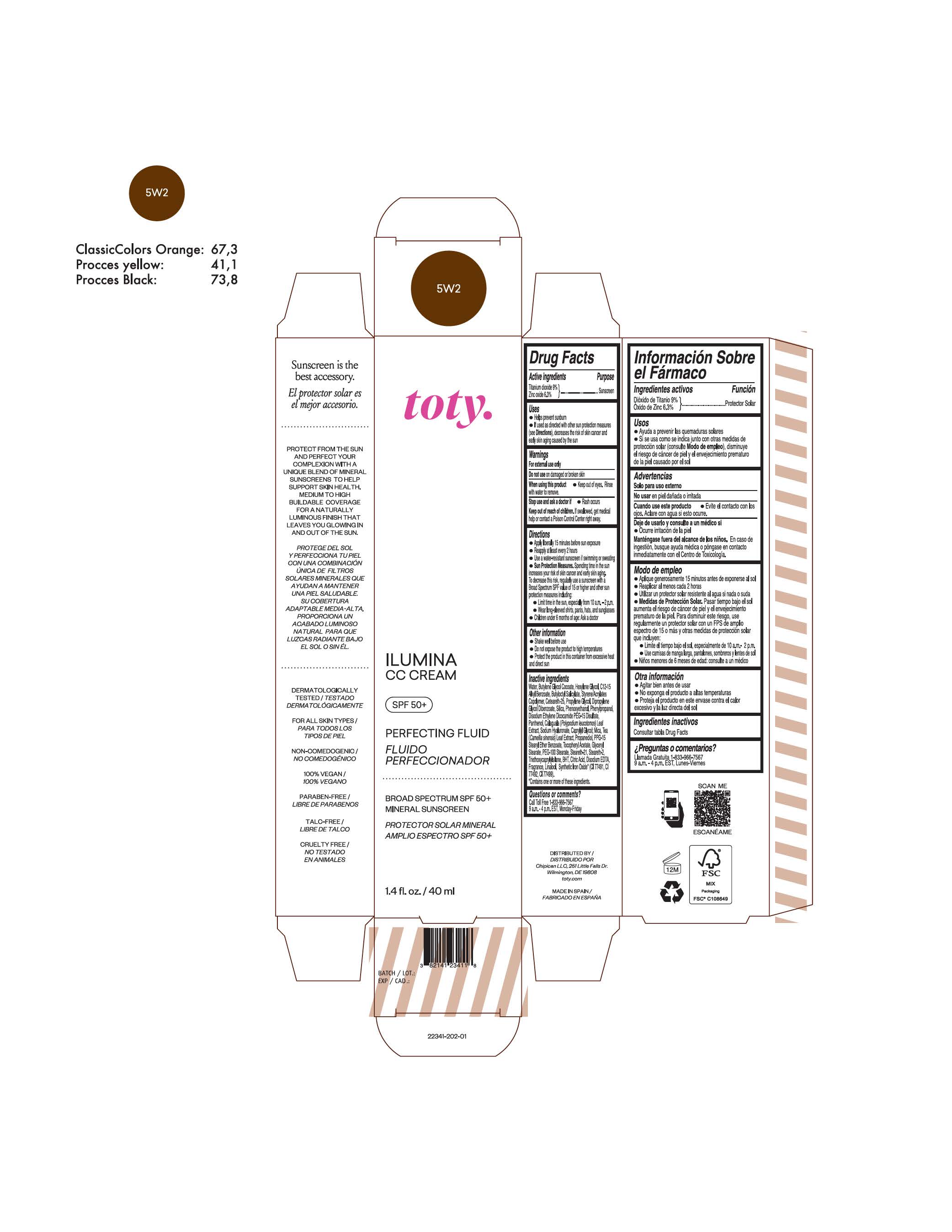
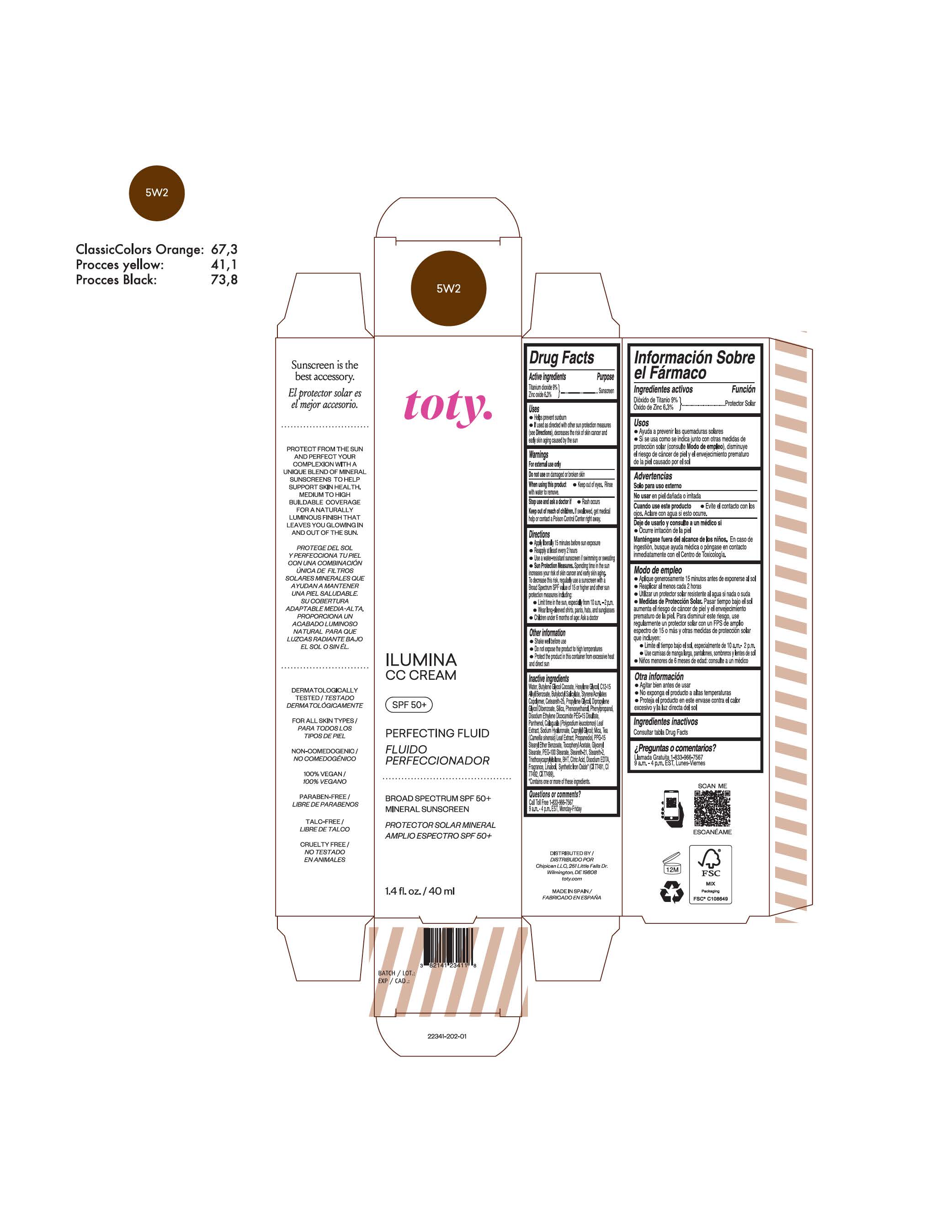
TOTY ILUMINA CC CREAM 5W2- titanium dioxide, zinc oxide cream
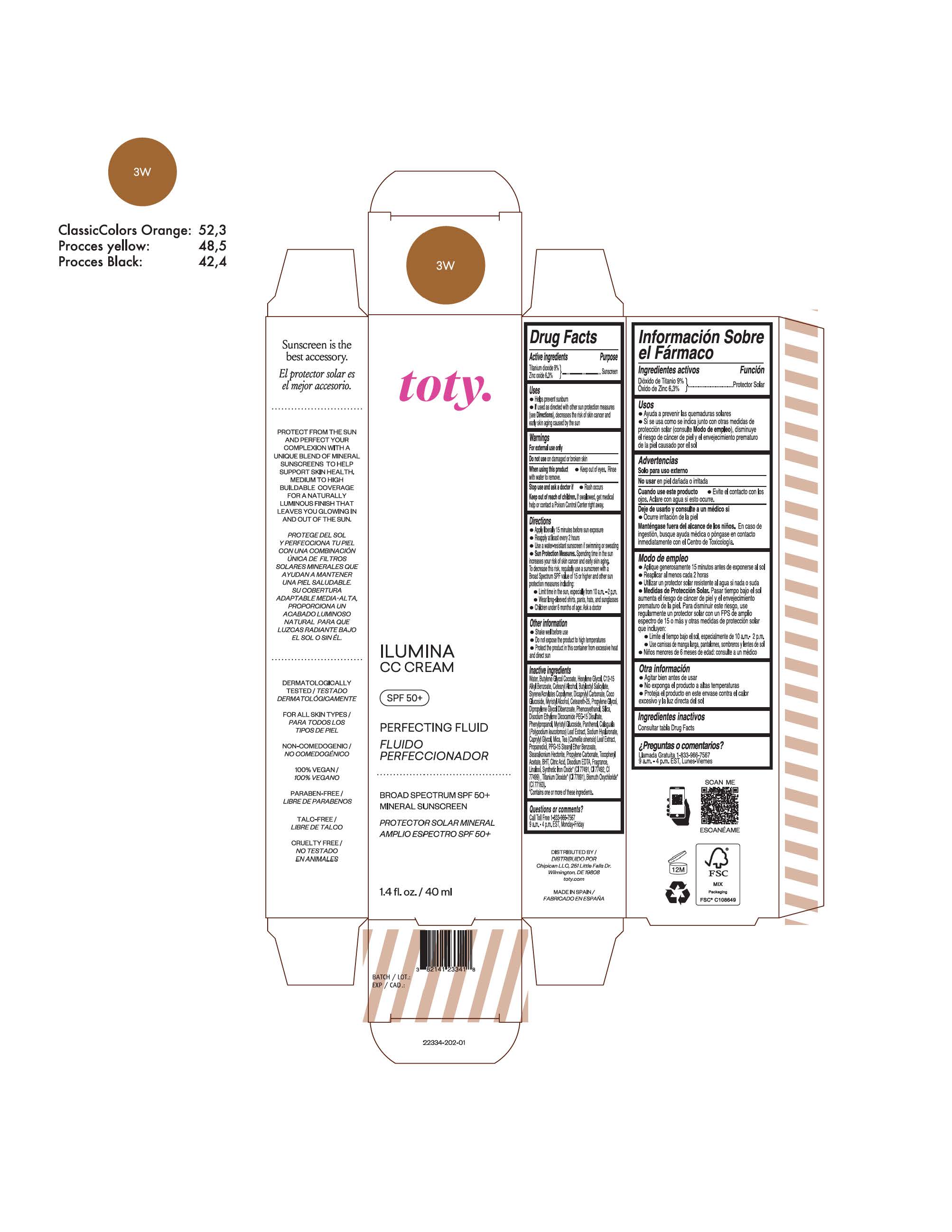
TOTY ILUMINA CC CREAM 3W- titanium dioxide, zinc oxide cream
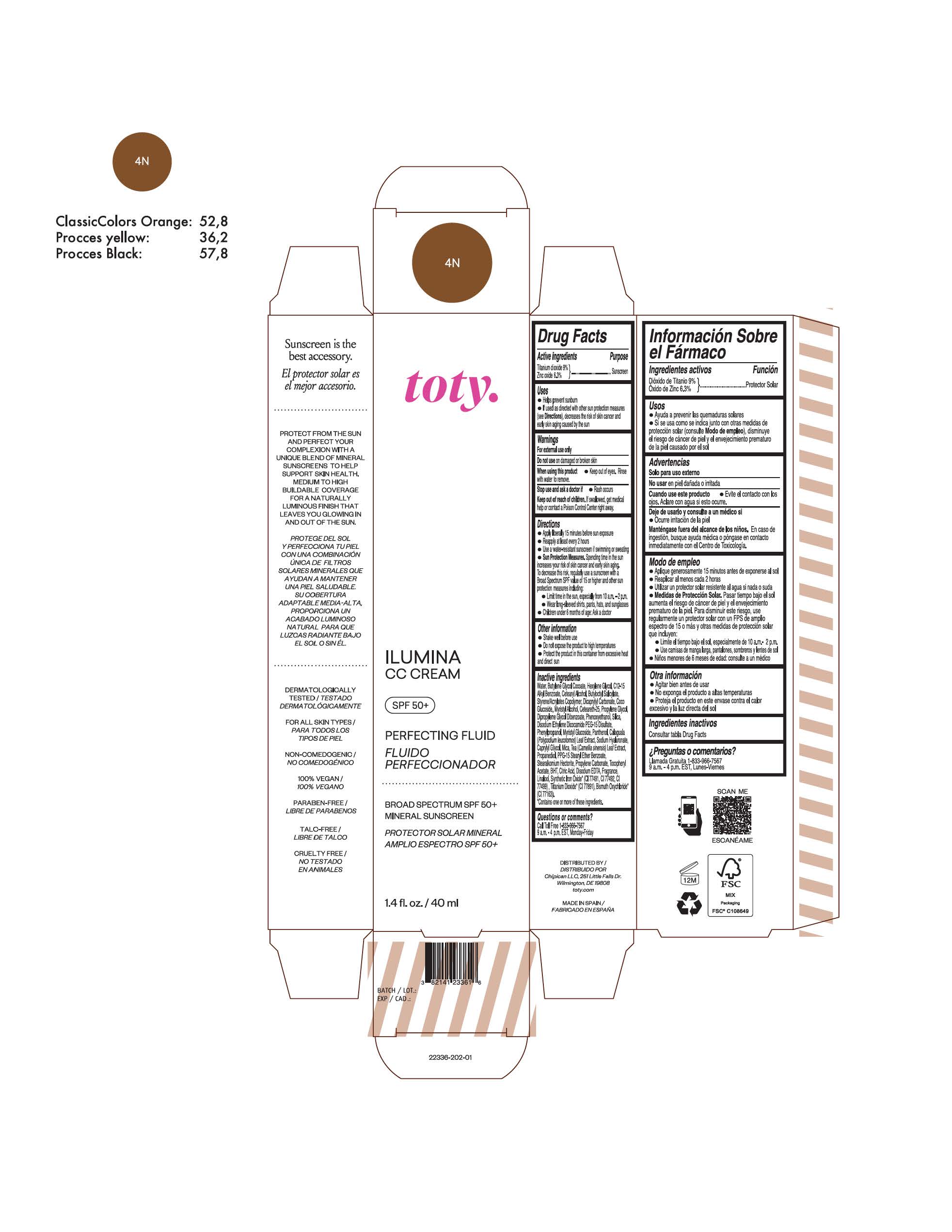
TOTY ILUMINA CC CREAM 4N- titanium dioxide, zinc oxide cream
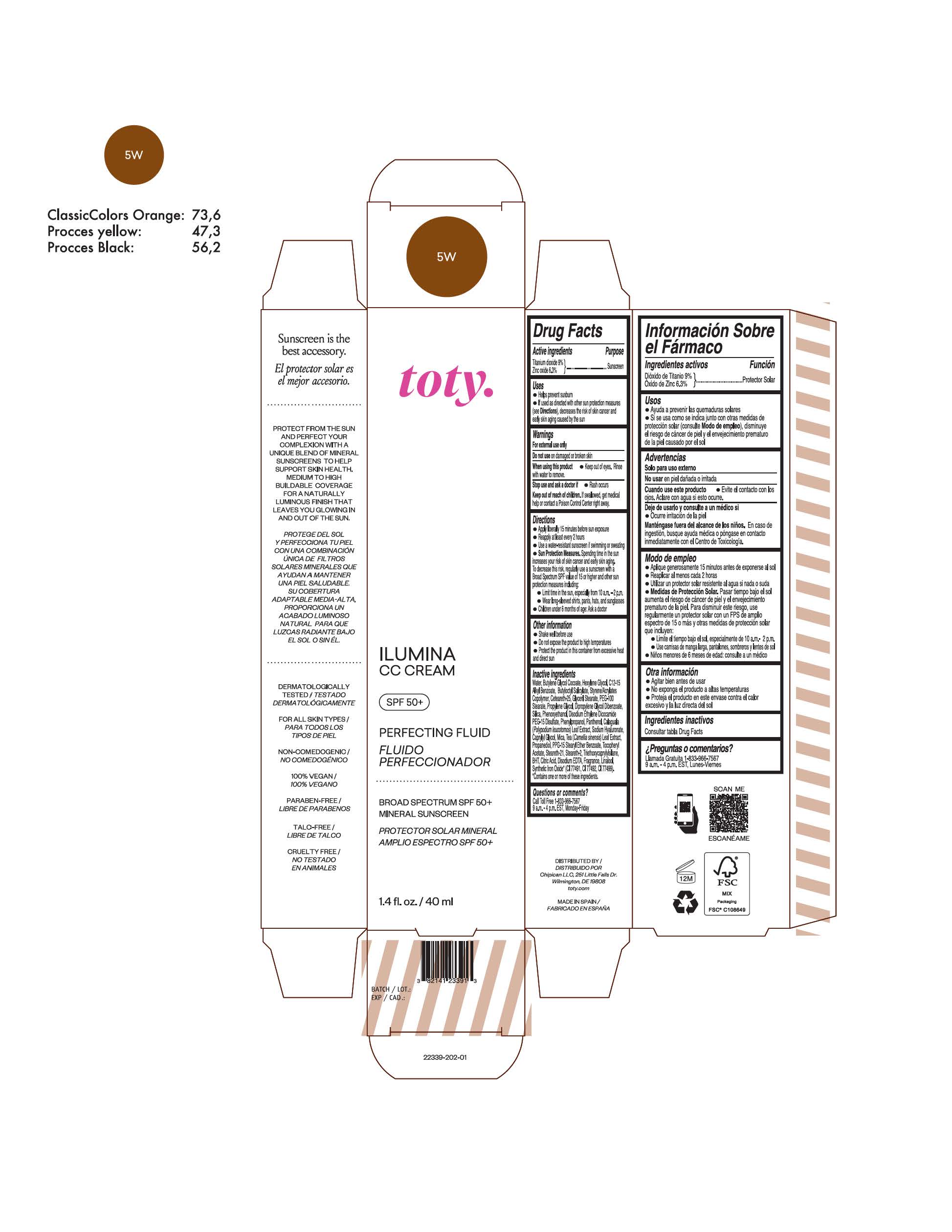
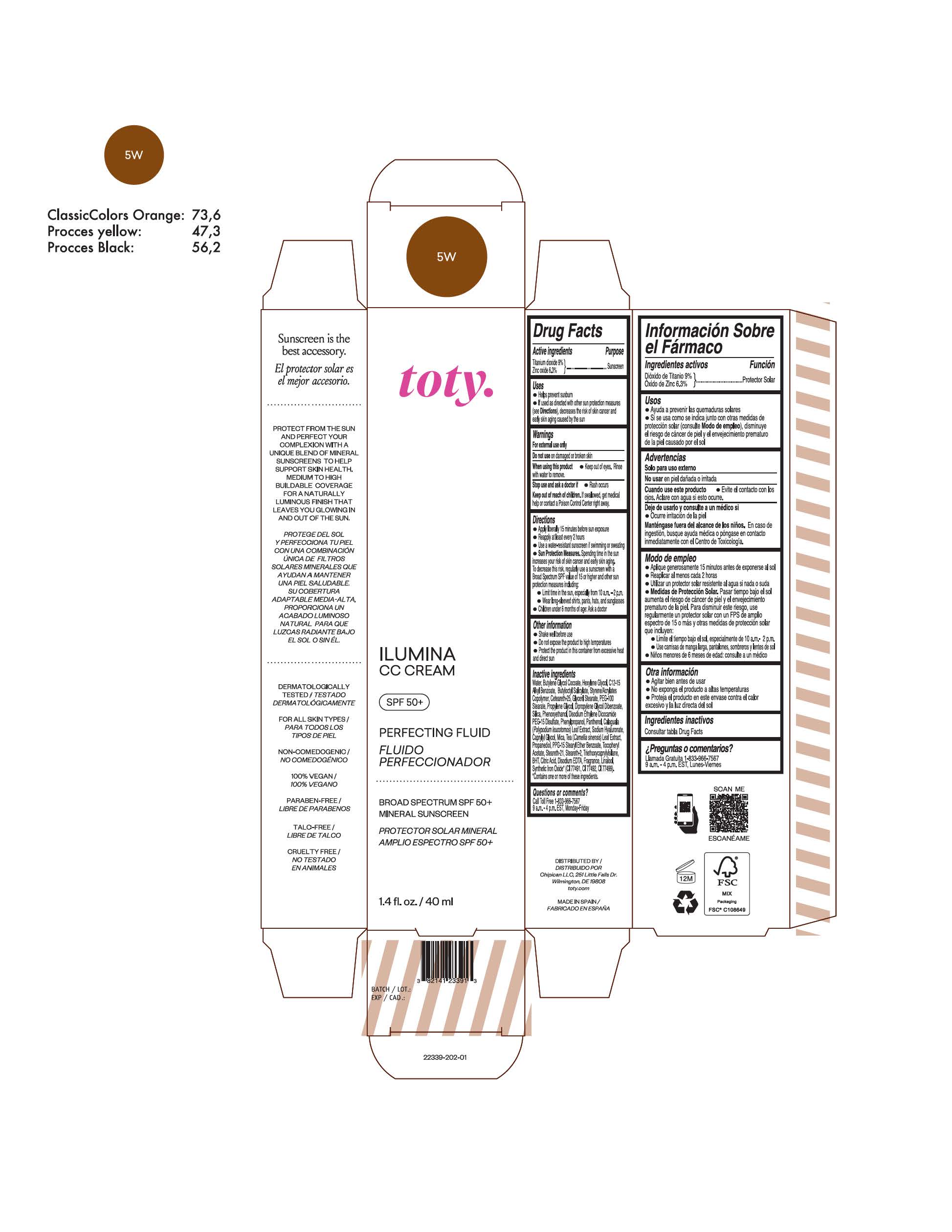
TOTY ILUMINA CC CREAM 5W- titanium dioxide, zinc oxide cream
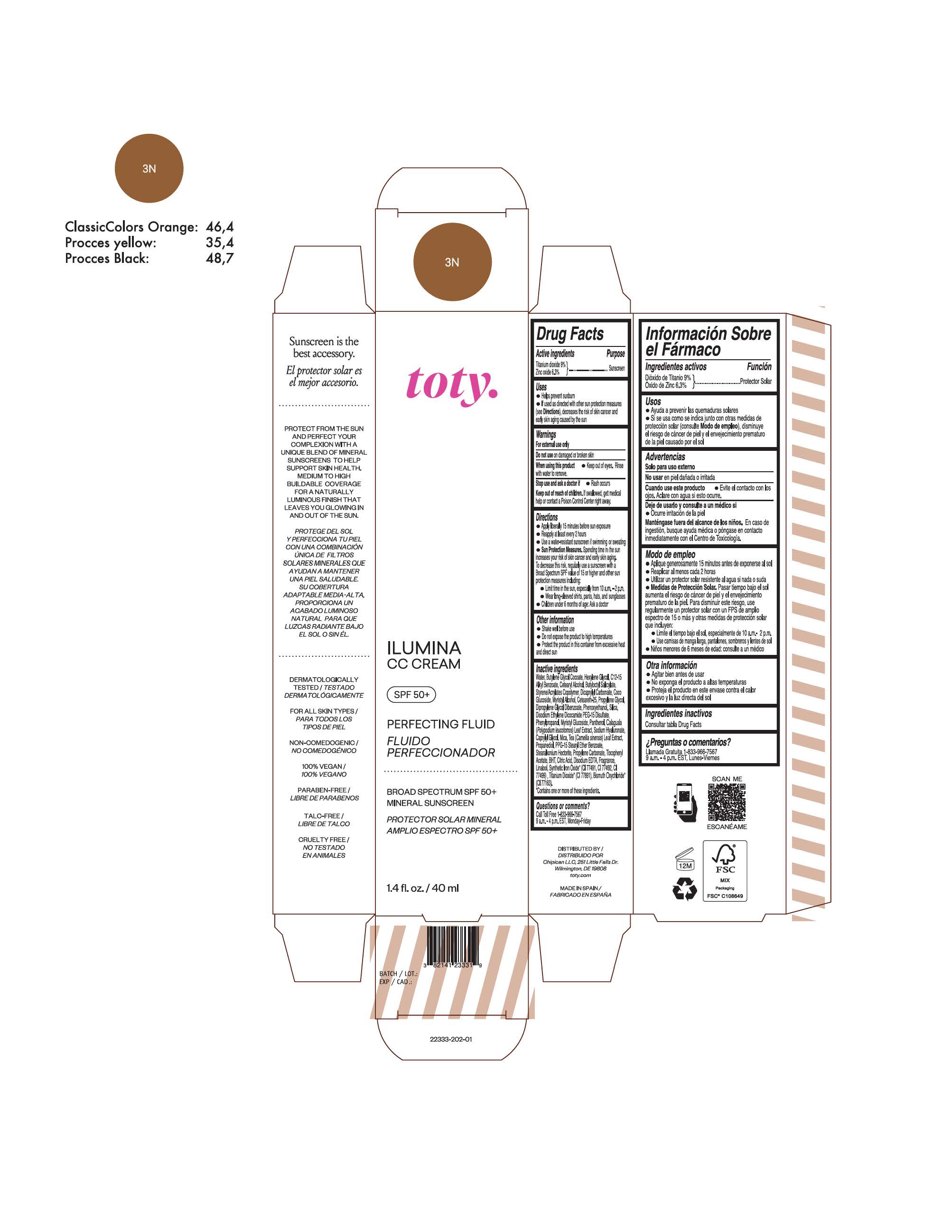
TOTY ILUMINA CC CREAM 3N- titanium dioxide, zinc oxide cream
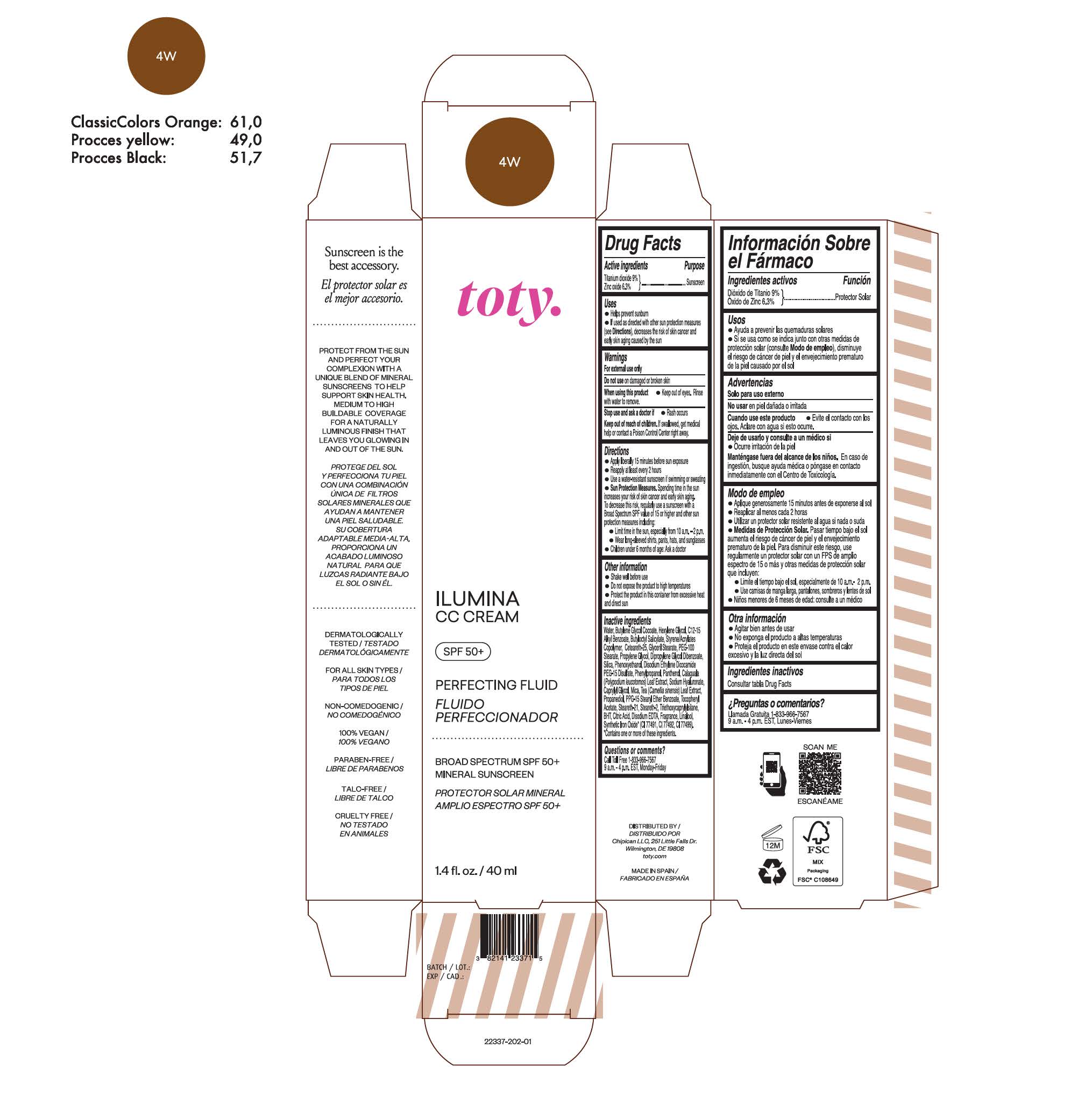
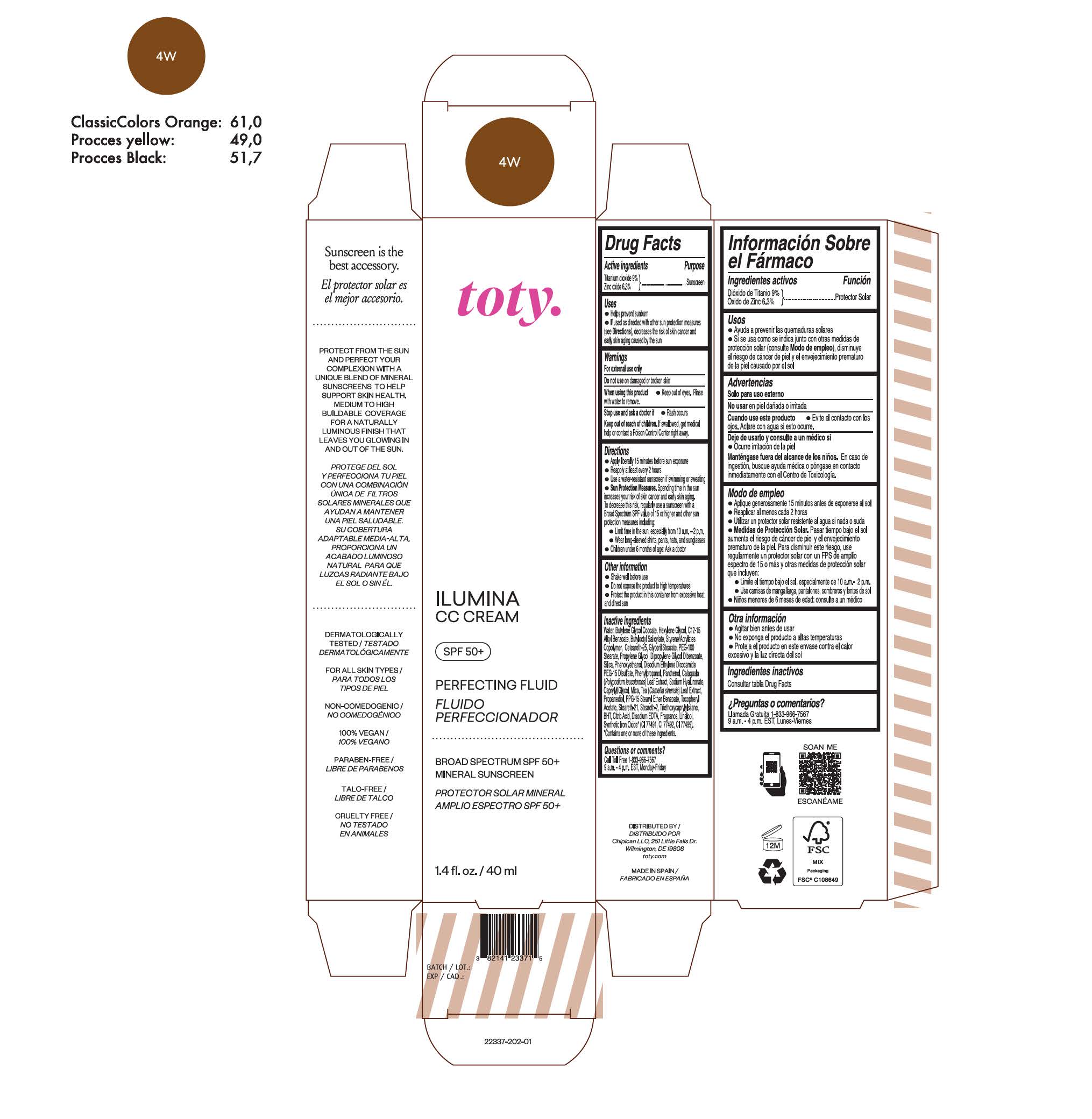
TOTY ILUMINA CC CREAM 4W- titanium dioxide, zinc oxide cream
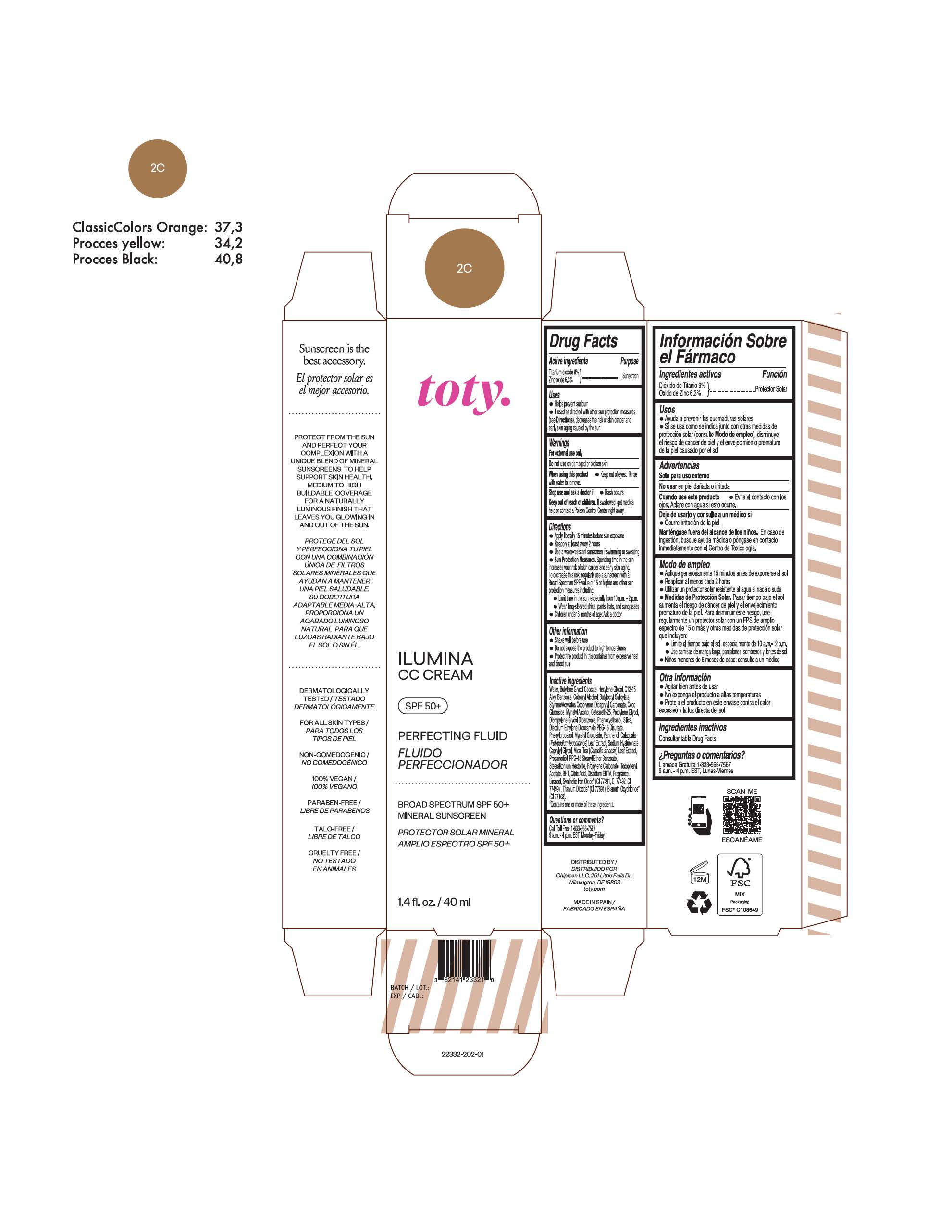
TOTY ILUMINA CC CREAM 2C- titanium dioxide, zinc oxide cream
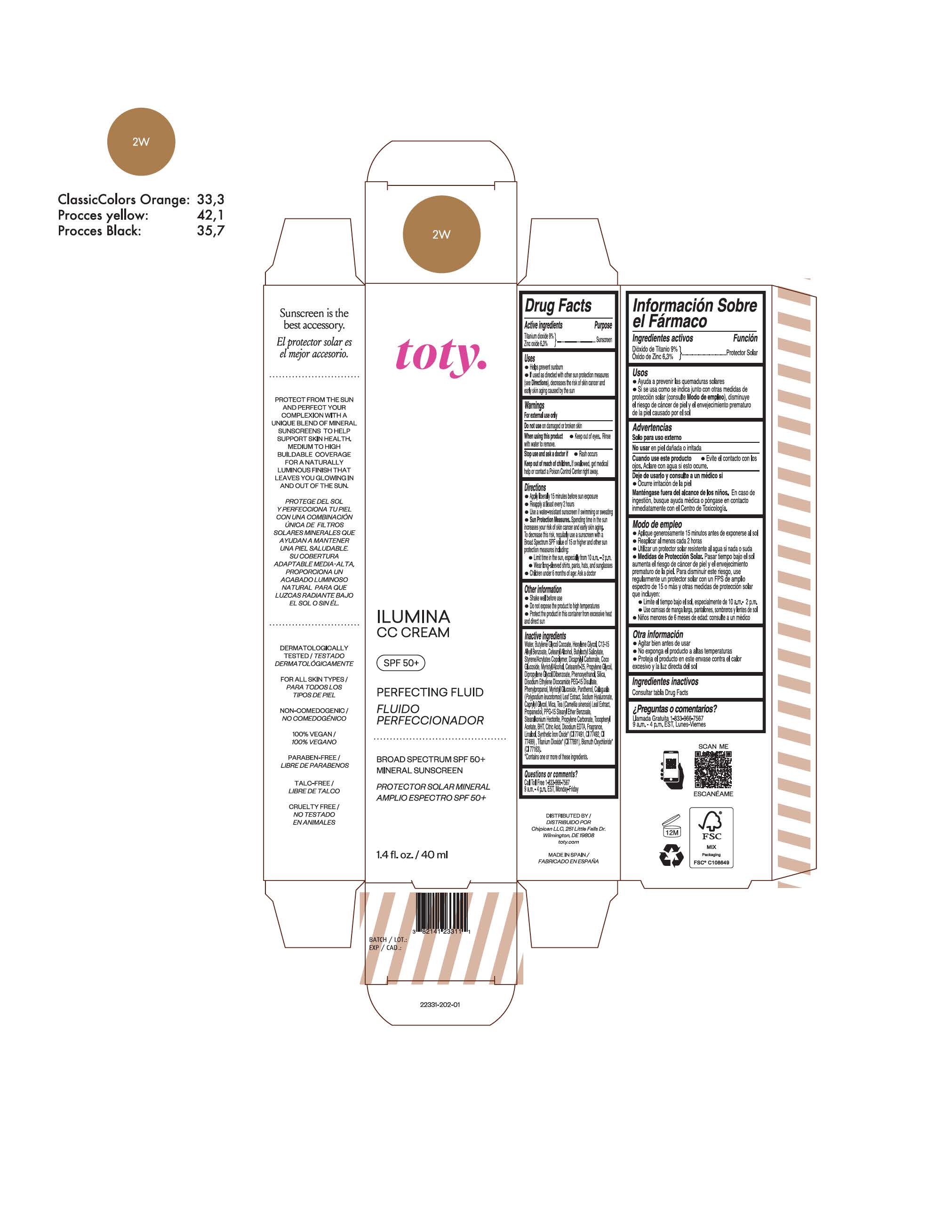
TOTY ILUMINA CC CREAM 2W- titanium dioxide, zinc oxide cream
-
NDC Code(s):
82141-2327-1,
82141-2328-1,
82141-2329-1,
82141-2330-1, view more82141-2331-1, 82141-2332-1, 82141-2333-1, 82141-2334-1, 82141-2335-1, 82141-2336-1, 82141-2337-1, 82141-2338-1, 82141-2339-1, 82141-2340-1, 82141-2341-1
- Packager: Chipican LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 19, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
- Uses
- Warnings
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
- Apply liberally 15 minutes behore sun exposure
- Reapply at least every 2 hours
- Use a water-resistant sunscreen if swimmimg or sweating
- Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- Limit time in the sun, especially from 10 a.m. - 2 p.m
- Wear long-sleeved shirts, pants, hats, and sunglasses
- Children under 6 months of age: Ask a doctor.
- Other Information
-
Inactive Ingredients
NDCs 82141-2327-1, 82141-2328-1
Water, Butylene Glycol Cocoate,Hexylene Glycol, C12-15
Alkyl Benzoate, Cetearyl Alcohol, Butyloctyl Salicylate,
Styrene/Acrylates Copolymer, Dicaprylyl Carbonate, Coco
Glucoside, Myristyl Alcohol, Ceteareth-25, Propylene Glycol,
Dipropylene Glycol Dibenzoate, Phenoxyethanol, Silica,
Disodium Ethylene Dicocamide PEG-15 Disulfate,
Phenylpropanol, Myristyl Glucoside, Panthenol, Calaguala
( Polypodium leucotomos) Leaf Extract, Sodium Hyaluronate,
Caprylyl Glycol, Tea ( Camellia sinensis) Leaf Extract,
Propanediol, PPG-15 Stearyl Ether Benzoate,
Stearalkonium Hectorite, Propylene Carbonate, Tocopheryl
Acetate, BHT, Citric Acid, Disodium EDTA, Fragrance,
Linalool, Synthetic Iron Oxide* (CI 77491, CI 77492, CI
77499), Titanium Dioxide* (CI 77891), Bismuth Oxychloride*
(CI 77163).
* Contains one or more of these ingredients.
NDCs 82141-2329-1, 82141-2330-1, 82141-2331-1, 82141-2332-1, 82141-2333-1
82141-2334-1, 82141-2336-1 & 82141-2338-1
Water, Butylene Glycol Cocoate,Hexylene Glycol, C12-15
Alkyl Benzoate, Cetearyl Alcohol, Butyloctyl Salicylate,
Styrene/Acrylates Copolymer, Dicaprylyl Carbonate, Coco
Glucoside, Myristyl Alcohol, Ceteareth-25, Propylene Glycol,
Dipropylene Glycol Dibenzoate, Phenoxyethanol, Silica,
Disodium Ethylene Dicocamide PEG-15 Disulfate,
Phenylpropanol, Myristyl Glucoside, Panthenol, Calaguala
( Polypodium leucotomos) Leaf Extract, Sodium Hyaluronate,
Caprylyl Glycol, Mica, Tea ( Camellia sinensis) Leaf Extract,
Propanediol, PPG-15 Stearyl Ether Benzoate,
Stearalkonium Hectorite, Propylene Carbonate, Tocopheryl
Acetate, BHT, Citric Acid, Disodium EDTA, Fragrance,
Linalool, Synthetic Iron Oxide* (CI 77491, CI 77492, CI
77499), Titanium Dioxide* (CI 77891), Bismuth Oxychloride*
(CI 77163).
* Contains one or more of these ingredients.
NDCs 82141-2335-1
Water, Butylene Glycol Cocoate,Hexylene Glycol, C12-15
Alkyl Benzoate, Cetearyl Alcohol, Butyloctyl Salicylate,
Styrene/Acrylates Copolymer, Dicaprylyl Carbonate, Coco
Glucoside, Myristyl Alcohol, Ceteareth-25, Propylene Glycol,
Dipropylene Glycol Dibenzoate, Phenoxyethanol, Silica,
Disodium Ethylene Dicocamide PEG-15 Disulfate,
Phenylpropanol, Myristyl Glucoside, Panthenol, Calaguala
( Polypodium leucotomos) Leaf Extract, Sodium Hyaluronate,
Caprylyl Glycol, Mica, Tea ( Camellia sinensis) Leaf Extract,
Propanediol, PPG-15 Stearyl Ether Benzoate,
Stearalkonium Hectorite, Propylene Carbonate, Tocopheryl
Acetate, BHT, Citric Acid, Disodium EDTA,
Triethoxycaprylylsilane, Fragrance, Linalool, Synthetic Iron
Oxide* (CI 77491, CI 77492, CI 77499), Titanium Dioxide*
(CI 77891), Bismuth Oxychloride* (CI 77163).
* Contains one or more of these ingredients.
NDCs 82141-2337-1 &82141-2339-1
Water, Butylene Glycol Cocoate,Hexylene Glycol, C12-15
Alkyl Benzoate, Butyloctyl Salicylate, Styrene/Acrylates
Copolymer, Ceteareth-25, Glyceril Stearate, PEG-100
Stearate, Propylene Glycol, Dipropylene Glycol Dibenzoate,
Silica, Phenoxyethanol, Disodium Ethylene Dicocamide
PEG-15 Disulfate, Phenylpropanol, Panthenol, Calaguala
( Polypodium leucotomos) Leaf Extract, Sodium Hyaluronate,
Caprylyl Glycol, Mica, Tea ( Camellia sinensis) Leaf Extract,
Propanediol, PPG-15 Stearyl Ether Benzoate, Tocopheryl
Acetate, Steareth-21, Steareth-2, Triethoxycaprylylsilane,
BHT, Citric Acid, Disodium EDTA, Fragrance, Linalool,
Synthetic Iron Oxide* (CI 77491, CI 77492, CI 77499).
* Contains one or more of these ingredients.
NDCs 82141-2340-1 & 82141-2341-1
Water, Butylene Glycol Cocoate,Hexylene Glycol, C12-15
Alkyl Benzoate, Butyloctyl Salicylate, Styrene/Acrylates
Copolymer, Ceteareth-25, Propylene Glycol, Dipropylene
Glycol Dibenzoate, Silica, Phenoxyethanol, Phenylpropanol,
Disodium Ethylene Dicocamide PEG-15 Disulfate,
Panthenol, Calaguala ( Polypodium leucotomos) Leaf
Extract, Sodium Hyaluronate, Caprylyl Glycol, Mica, Tea
( Camellia sinensis) Leaf Extract, Propanediol, PPG-15
Stearyl Ether Benzoate, Tocopheryl Acetate, Glyceryl
Stearate, PEG-100 Stearate, Steareth-21, Steareth-2,
Triethoxycaprylylsilane, BHT, Citric Acid, Disodium EDTA,
Fragrance, Linalool, Synthetic Iron Oxide* (CI 77491, CI
77492, CI 77499).
* Contains one or more of these ingredients.
- toty Ilumina CC Cream 1N
- toty Ilumina CC Cream 1W
- toty Ilumina CC Cream 1C
- toty Ilumina CC Cream 2N
- toty Ilumina CC Cream 2W
- toty Ilumina CC Cream 2C
- toty Ilumina CC Cream 3N
- toty Ilumina CC Cream 3W
- toty Ilumina CC Cream 3C
- toty Ilumina CC Cream 4N
- toty Ilumina CC Cream 4W
- toty Ilumina CC Cream 4C
- toty Ilumina CC Cream 5W
- toty Ilumina CC Cream 5W1
- toty Ilumina CC Cream 5W2
-
INGREDIENTS AND APPEARANCE
TOTY ILUMINA CC CREAM 1N
titanium dioxide, zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82141-2327 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 9 g in 100 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 6.3 g in 100 g Inactive Ingredients Ingredient Name Strength 1,3-BUTYLENE GLYCOL COCOATE (UNII: B2JUC5N9EH) BUTYL METHACRYLATE/METHYL METHACRYLATE/METHACRYLIC ACID/STYRENE CROSSPOLYMER (UNII: V5RS026Q0H) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) CAPRYLYL GLYCOL (UNII: 00YIU5438U) GREEN TEA LEAF (UNII: W2ZU1RY8B0) STEARALKONIUM HECTORITE (UNII: OLX698AH5P) PROPYLENE CARBONATE (UNII: 8D08K3S51E) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PHLEBODIUM AUREUM LEAF (UNII: BG58BW230G) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) COCO GLUCOSIDE (UNII: ICS790225B) WATER (UNII: 059QF0KO0R) HEXYLENE GLYCOL (UNII: KEH0A3F75J) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) CETEARETH-25 (UNII: 8FA93U5T67) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) LINALOOL, (+/-)- (UNII: D81QY6I88E) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) HYALURONATE SODIUM (UNII: YSE9PPT4TH) DICAPRYLYL CARBONATE (UNII: 609A3V1SUA) DIPROPYLENE GLYCOL DIBENZOATE (UNII: 6OA5ZDY41O) PROPANEDIOL (UNII: 5965N8W85T) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) PHENOXYETHANOL (UNII: HIE492ZZ3T) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) DISODIUM ETHYLENE DICOCAMIDE PEG-15 DISULFATE (UNII: QI9A6U005W) PPG-15 STEARYL ETHER BENZOATE (UNII: 80D2J6361M) PHENYLPROPANOL (UNII: 0F897O3O4M) MYRISTYL ALCOHOL (UNII: V42034O9PU) MYRISTYL GLUCOSIDE (UNII: 6AK28695LF) PANTHENOL (UNII: WV9CM0O67Z) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82141-2327-1 1 in 1 CARTON 10/01/2022 1 40 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 10/01/2022 TOTY ILUMINA CC CREAM 4C
titanium dioxide, zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82141-2338 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 9 g in 100 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 6.3 g in 100 g Inactive Ingredients Ingredient Name Strength BUTYL METHACRYLATE/METHYL METHACRYLATE/METHACRYLIC ACID/STYRENE CROSSPOLYMER (UNII: V5RS026Q0H) 1,3-BUTYLENE GLYCOL COCOATE (UNII: B2JUC5N9EH) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CAPRYLYL GLYCOL (UNII: 00YIU5438U) GREEN TEA LEAF (UNII: W2ZU1RY8B0) STEARALKONIUM HECTORITE (UNII: OLX698AH5P) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PHLEBODIUM AUREUM LEAF (UNII: BG58BW230G) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) COCO GLUCOSIDE (UNII: ICS790225B) WATER (UNII: 059QF0KO0R) HEXYLENE GLYCOL (UNII: KEH0A3F75J) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) CETEARETH-25 (UNII: 8FA93U5T67) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) LINALOOL, (+/-)- (UNII: D81QY6I88E) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) HYALURONATE SODIUM (UNII: YSE9PPT4TH) DICAPRYLYL CARBONATE (UNII: 609A3V1SUA) DIPROPYLENE GLYCOL DIBENZOATE (UNII: 6OA5ZDY41O) PROPANEDIOL (UNII: 5965N8W85T) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) PHENOXYETHANOL (UNII: HIE492ZZ3T) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) DISODIUM ETHYLENE DICOCAMIDE PEG-15 DISULFATE (UNII: QI9A6U005W) PPG-15 STEARYL ETHER BENZOATE (UNII: 80D2J6361M) PHENYLPROPANOL (UNII: 0F897O3O4M) MYRISTYL ALCOHOL (UNII: V42034O9PU) MYRISTYL GLUCOSIDE (UNII: 6AK28695LF) PANTHENOL (UNII: WV9CM0O67Z) PROPYLENE CARBONATE (UNII: 8D08K3S51E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82141-2338-1 1 in 1 CARTON 10/01/2022 1 40 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 10/01/2022 TOTY ILUMINA CC CREAM 1C
titanium dioxide, zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82141-2329 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 9 g in 100 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 6.3 g in 100 g Inactive Ingredients Ingredient Name Strength BUTYL METHACRYLATE/METHYL METHACRYLATE/METHACRYLIC ACID/STYRENE CROSSPOLYMER (UNII: V5RS026Q0H) 1,3-BUTYLENE GLYCOL COCOATE (UNII: B2JUC5N9EH) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CAPRYLYL GLYCOL (UNII: 00YIU5438U) GREEN TEA LEAF (UNII: W2ZU1RY8B0) STEARALKONIUM HECTORITE (UNII: OLX698AH5P) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PHLEBODIUM AUREUM LEAF (UNII: BG58BW230G) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) COCO GLUCOSIDE (UNII: ICS790225B) WATER (UNII: 059QF0KO0R) HEXYLENE GLYCOL (UNII: KEH0A3F75J) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) CETEARETH-25 (UNII: 8FA93U5T67) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) LINALOOL, (+/-)- (UNII: D81QY6I88E) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) HYALURONATE SODIUM (UNII: YSE9PPT4TH) DICAPRYLYL CARBONATE (UNII: 609A3V1SUA) DIPROPYLENE GLYCOL DIBENZOATE (UNII: 6OA5ZDY41O) PROPANEDIOL (UNII: 5965N8W85T) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) PHENOXYETHANOL (UNII: HIE492ZZ3T) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) DISODIUM ETHYLENE DICOCAMIDE PEG-15 DISULFATE (UNII: QI9A6U005W) PPG-15 STEARYL ETHER BENZOATE (UNII: 80D2J6361M) PHENYLPROPANOL (UNII: 0F897O3O4M) MYRISTYL ALCOHOL (UNII: V42034O9PU) MYRISTYL GLUCOSIDE (UNII: 6AK28695LF) PANTHENOL (UNII: WV9CM0O67Z) PROPYLENE CARBONATE (UNII: 8D08K3S51E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82141-2329-1 1 in 1 CARTON 10/01/2022 1 40 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 10/01/2022 TOTY ILUMINA CC CREAM 1W
titanium dioxide, zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82141-2328 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 9 g in 100 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 6.3 g in 100 g Inactive Ingredients Ingredient Name Strength BUTYL METHACRYLATE/METHYL METHACRYLATE/METHACRYLIC ACID/STYRENE CROSSPOLYMER (UNII: V5RS026Q0H) 1,3-BUTYLENE GLYCOL COCOATE (UNII: B2JUC5N9EH) CAPRYLYL GLYCOL (UNII: 00YIU5438U) GREEN TEA LEAF (UNII: W2ZU1RY8B0) STEARALKONIUM HECTORITE (UNII: OLX698AH5P) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PHLEBODIUM AUREUM LEAF (UNII: BG58BW230G) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) COCO GLUCOSIDE (UNII: ICS790225B) WATER (UNII: 059QF0KO0R) HEXYLENE GLYCOL (UNII: KEH0A3F75J) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) CETEARETH-25 (UNII: 8FA93U5T67) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) LINALOOL, (+/-)- (UNII: D81QY6I88E) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) HYALURONATE SODIUM (UNII: YSE9PPT4TH) DICAPRYLYL CARBONATE (UNII: 609A3V1SUA) DIPROPYLENE GLYCOL DIBENZOATE (UNII: 6OA5ZDY41O) PROPANEDIOL (UNII: 5965N8W85T) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) PHENOXYETHANOL (UNII: HIE492ZZ3T) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) DISODIUM ETHYLENE DICOCAMIDE PEG-15 DISULFATE (UNII: QI9A6U005W) PPG-15 STEARYL ETHER BENZOATE (UNII: 80D2J6361M) PHENYLPROPANOL (UNII: 0F897O3O4M) MYRISTYL ALCOHOL (UNII: V42034O9PU) MYRISTYL GLUCOSIDE (UNII: 6AK28695LF) PANTHENOL (UNII: WV9CM0O67Z) PROPYLENE CARBONATE (UNII: 8D08K3S51E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82141-2328-1 1 in 1 CARTON 10/01/2022 1 40 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 10/01/2022 TOTY ILUMINA CC CREAM 2N
titanium dioxide, zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82141-2330 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 9 g in 100 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 6.3 g in 100 g Inactive Ingredients Ingredient Name Strength BUTYL METHACRYLATE/METHYL METHACRYLATE/METHACRYLIC ACID/STYRENE CROSSPOLYMER (UNII: V5RS026Q0H) 1,3-BUTYLENE GLYCOL COCOATE (UNII: B2JUC5N9EH) CAPRYLYL GLYCOL (UNII: 00YIU5438U) GREEN TEA LEAF (UNII: W2ZU1RY8B0) STEARALKONIUM HECTORITE (UNII: OLX698AH5P) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PHLEBODIUM AUREUM LEAF (UNII: BG58BW230G) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) COCO GLUCOSIDE (UNII: ICS790225B) WATER (UNII: 059QF0KO0R) HEXYLENE GLYCOL (UNII: KEH0A3F75J) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) CETEARETH-25 (UNII: 8FA93U5T67) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) LINALOOL, (+/-)- (UNII: D81QY6I88E) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) HYALURONATE SODIUM (UNII: YSE9PPT4TH) DICAPRYLYL CARBONATE (UNII: 609A3V1SUA) DIPROPYLENE GLYCOL DIBENZOATE (UNII: 6OA5ZDY41O) PROPANEDIOL (UNII: 5965N8W85T) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) PHENOXYETHANOL (UNII: HIE492ZZ3T) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) DISODIUM ETHYLENE DICOCAMIDE PEG-15 DISULFATE (UNII: QI9A6U005W) PPG-15 STEARYL ETHER BENZOATE (UNII: 80D2J6361M) PHENYLPROPANOL (UNII: 0F897O3O4M) MYRISTYL ALCOHOL (UNII: V42034O9PU) MYRISTYL GLUCOSIDE (UNII: 6AK28695LF) PANTHENOL (UNII: WV9CM0O67Z) PROPYLENE CARBONATE (UNII: 8D08K3S51E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82141-2330-1 1 in 1 CARTON 10/01/2022 1 40 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 10/01/2022 TOTY ILUMINA CC CREAM 3C
titanium dioxide, zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82141-2335 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 9 g in 100 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 6.3 g in 100 g Inactive Ingredients Ingredient Name Strength 1,3-BUTYLENE GLYCOL COCOATE (UNII: B2JUC5N9EH) BUTYL METHACRYLATE/METHYL METHACRYLATE/METHACRYLIC ACID/STYRENE CROSSPOLYMER (UNII: V5RS026Q0H) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CAPRYLYL GLYCOL (UNII: 00YIU5438U) GREEN TEA LEAF (UNII: W2ZU1RY8B0) STEARALKONIUM HECTORITE (UNII: OLX698AH5P) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PHLEBODIUM AUREUM LEAF (UNII: BG58BW230G) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) COCO GLUCOSIDE (UNII: ICS790225B) WATER (UNII: 059QF0KO0R) HEXYLENE GLYCOL (UNII: KEH0A3F75J) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) BISMUTH OXYCHLORIDE (UNII: 4ZR792I587) CETEARETH-25 (UNII: 8FA93U5T67) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) LINALOOL, (+/-)- (UNII: D81QY6I88E) FERRIC OXIDE RED (UNII: 1K09F3G675) HYALURONATE SODIUM (UNII: YSE9PPT4TH) DICAPRYLYL CARBONATE (UNII: 609A3V1SUA) DIPROPYLENE GLYCOL DIBENZOATE (UNII: 6OA5ZDY41O) PROPANEDIOL (UNII: 5965N8W85T) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) PHENOXYETHANOL (UNII: HIE492ZZ3T) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) DISODIUM ETHYLENE DICOCAMIDE PEG-15 DISULFATE (UNII: QI9A6U005W) PPG-15 STEARYL ETHER BENZOATE (UNII: 80D2J6361M) PHENYLPROPANOL (UNII: 0F897O3O4M) MYRISTYL ALCOHOL (UNII: V42034O9PU) MYRISTYL GLUCOSIDE (UNII: 6AK28695LF) PANTHENOL (UNII: WV9CM0O67Z) PROPYLENE CARBONATE (UNII: 8D08K3S51E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82141-2335-1 1 in 1 CARTON 10/01/2022 1 40 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 10/01/2022 TOTY ILUMINA CC CREAM 5W1
titanium dioxide, zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82141-2340 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 9 g in 100 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 6.3 g in 100 g Inactive Ingredients Ingredient Name Strength BUTYL METHACRYLATE/METHYL METHACRYLATE/METHACRYLIC ACID/STYRENE CROSSPOLYMER (UNII: V5RS026Q0H) 1,3-BUTYLENE GLYCOL COCOATE (UNII: B2JUC5N9EH) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) PEG-100 STEARATE (UNII: YD01N1999R) MICA (UNII: V8A1AW0880) STEARETH-21 (UNII: 53J3F32P58) STEARETH-2 (UNII: V56DFE46J5) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CAPRYLYL GLYCOL (UNII: 00YIU5438U) GREEN TEA LEAF (UNII: W2ZU1RY8B0) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PHLEBODIUM AUREUM LEAF (UNII: BG58BW230G) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) HEXYLENE GLYCOL (UNII: KEH0A3F75J) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) CETEARETH-25 (UNII: 8FA93U5T67) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) LINALOOL, (+/-)- (UNII: D81QY6I88E) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) HYALURONATE SODIUM (UNII: YSE9PPT4TH) DIPROPYLENE GLYCOL DIBENZOATE (UNII: 6OA5ZDY41O) PROPANEDIOL (UNII: 5965N8W85T) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) PHENOXYETHANOL (UNII: HIE492ZZ3T) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) DISODIUM ETHYLENE DICOCAMIDE PEG-15 DISULFATE (UNII: QI9A6U005W) PPG-15 STEARYL ETHER BENZOATE (UNII: 80D2J6361M) PHENYLPROPANOL (UNII: 0F897O3O4M) PANTHENOL (UNII: WV9CM0O67Z) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82141-2340-1 1 in 1 CARTON 10/01/2022 1 40 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 10/01/2022 TOTY ILUMINA CC CREAM 5W2
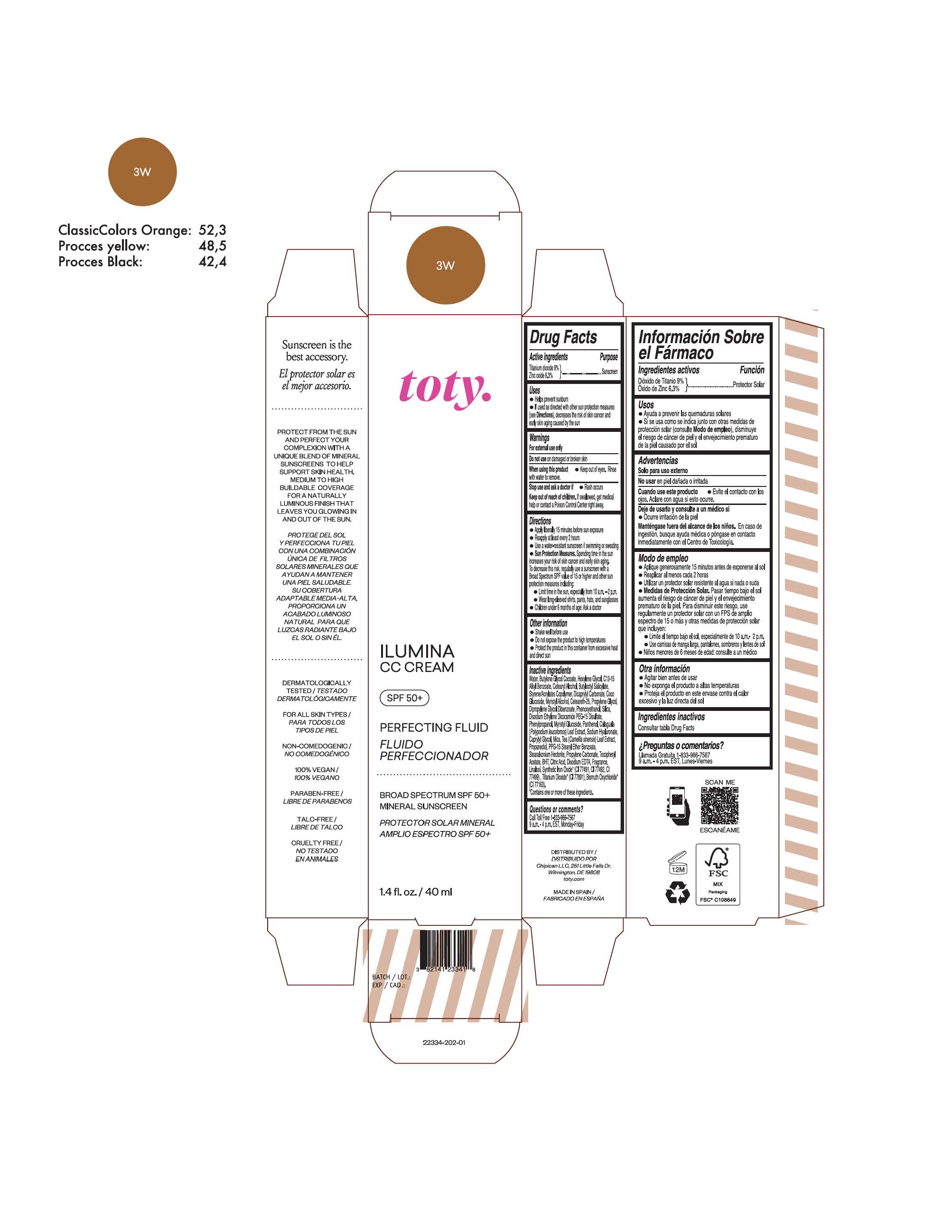
titanium dioxide, zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82141-2341 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 9 g in 100 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 6.3 g in 100 g Inactive Ingredients Ingredient Name Strength BUTYL METHACRYLATE/METHYL METHACRYLATE/METHACRYLIC ACID/STYRENE CROSSPOLYMER (UNII: V5RS026Q0H) 1,3-BUTYLENE GLYCOL COCOATE (UNII: B2JUC5N9EH) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) PEG-100 STEARATE (UNII: YD01N1999R) MICA (UNII: V8A1AW0880) STEARETH-21 (UNII: 53J3F32P58) STEARETH-2 (UNII: V56DFE46J5) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CAPRYLYL GLYCOL (UNII: 00YIU5438U) GREEN TEA LEAF (UNII: W2ZU1RY8B0) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PHLEBODIUM AUREUM LEAF (UNII: BG58BW230G) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) HEXYLENE GLYCOL (UNII: KEH0A3F75J) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) CETEARETH-25 (UNII: 8FA93U5T67) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) LINALOOL, (+/-)- (UNII: D81QY6I88E) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) HYALURONATE SODIUM (UNII: YSE9PPT4TH) DIPROPYLENE GLYCOL DIBENZOATE (UNII: 6OA5ZDY41O) PROPANEDIOL (UNII: 5965N8W85T) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) PHENOXYETHANOL (UNII: HIE492ZZ3T) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) DISODIUM ETHYLENE DICOCAMIDE PEG-15 DISULFATE (UNII: QI9A6U005W) PPG-15 STEARYL ETHER BENZOATE (UNII: 80D2J6361M) PHENYLPROPANOL (UNII: 0F897O3O4M) PANTHENOL (UNII: WV9CM0O67Z) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82141-2341-1 1 in 1 CARTON 10/01/2022 1 40 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 10/01/2022 TOTY ILUMINA CC CREAM 3W
titanium dioxide, zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82141-2334 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 9 g in 100 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 6.3 g in 100 g Inactive Ingredients Ingredient Name Strength 1,3-BUTYLENE GLYCOL COCOATE (UNII: B2JUC5N9EH) BUTYL METHACRYLATE/METHYL METHACRYLATE/METHACRYLIC ACID/STYRENE CROSSPOLYMER (UNII: V5RS026Q0H) STEARALKONIUM HECTORITE (UNII: OLX698AH5P) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CAPRYLYL GLYCOL (UNII: 00YIU5438U) GREEN TEA LEAF (UNII: W2ZU1RY8B0) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PHLEBODIUM AUREUM LEAF (UNII: BG58BW230G) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) COCO GLUCOSIDE (UNII: ICS790225B) WATER (UNII: 059QF0KO0R) HEXYLENE GLYCOL (UNII: KEH0A3F75J) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) CETEARETH-25 (UNII: 8FA93U5T67) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) LINALOOL, (+/-)- (UNII: D81QY6I88E) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) HYALURONATE SODIUM (UNII: YSE9PPT4TH) DICAPRYLYL CARBONATE (UNII: 609A3V1SUA) DIPROPYLENE GLYCOL DIBENZOATE (UNII: 6OA5ZDY41O) PROPANEDIOL (UNII: 5965N8W85T) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) PHENOXYETHANOL (UNII: HIE492ZZ3T) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MYRISTYL ALCOHOL (UNII: V42034O9PU) DISODIUM ETHYLENE DICOCAMIDE PEG-15 DISULFATE (UNII: QI9A6U005W) PPG-15 STEARYL ETHER BENZOATE (UNII: 80D2J6361M) PHENYLPROPANOL (UNII: 0F897O3O4M) MYRISTYL GLUCOSIDE (UNII: 6AK28695LF) PANTHENOL (UNII: WV9CM0O67Z) PROPYLENE CARBONATE (UNII: 8D08K3S51E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82141-2334-1 1 in 1 CARTON 10/01/2022 1 40 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 10/01/2022 TOTY ILUMINA CC CREAM 4N
titanium dioxide, zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82141-2336 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 9 g in 100 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 6.3 g in 100 g Inactive Ingredients Ingredient Name Strength BUTYL METHACRYLATE/METHYL METHACRYLATE/METHACRYLIC ACID/STYRENE CROSSPOLYMER (UNII: V5RS026Q0H) 1,3-BUTYLENE GLYCOL COCOATE (UNII: B2JUC5N9EH) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CAPRYLYL GLYCOL (UNII: 00YIU5438U) GREEN TEA LEAF (UNII: W2ZU1RY8B0) STEARALKONIUM HECTORITE (UNII: OLX698AH5P) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PHLEBODIUM AUREUM LEAF (UNII: BG58BW230G) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) COCO GLUCOSIDE (UNII: ICS790225B) WATER (UNII: 059QF0KO0R) HEXYLENE GLYCOL (UNII: KEH0A3F75J) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) CETEARETH-25 (UNII: 8FA93U5T67) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) LINALOOL, (+/-)- (UNII: D81QY6I88E) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) HYALURONATE SODIUM (UNII: YSE9PPT4TH) DICAPRYLYL CARBONATE (UNII: 609A3V1SUA) DIPROPYLENE GLYCOL DIBENZOATE (UNII: 6OA5ZDY41O) PROPANEDIOL (UNII: 5965N8W85T) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) PHENOXYETHANOL (UNII: HIE492ZZ3T) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) DISODIUM ETHYLENE DICOCAMIDE PEG-15 DISULFATE (UNII: QI9A6U005W) PPG-15 STEARYL ETHER BENZOATE (UNII: 80D2J6361M) PHENYLPROPANOL (UNII: 0F897O3O4M) MYRISTYL ALCOHOL (UNII: V42034O9PU) MYRISTYL GLUCOSIDE (UNII: 6AK28695LF) PANTHENOL (UNII: WV9CM0O67Z) PROPYLENE CARBONATE (UNII: 8D08K3S51E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82141-2336-1 1 in 1 CARTON 10/01/2022 1 40 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 10/01/2022 TOTY ILUMINA CC CREAM 5W
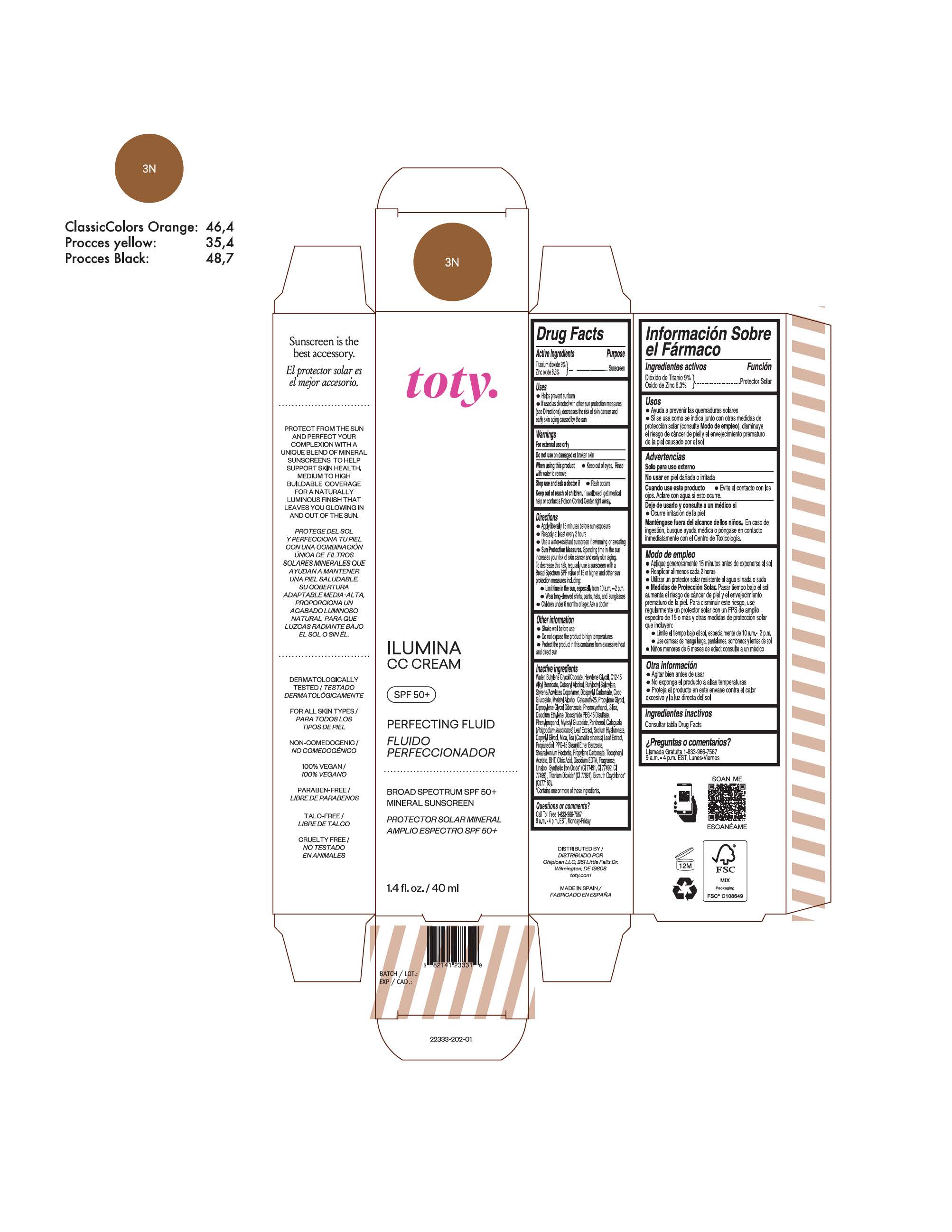
titanium dioxide, zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82141-2339 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 9 g in 100 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 6.3 g in 100 g Inactive Ingredients Ingredient Name Strength 1,3-BUTYLENE GLYCOL COCOATE (UNII: B2JUC5N9EH) BUTYL METHACRYLATE/METHYL METHACRYLATE/METHACRYLIC ACID/STYRENE CROSSPOLYMER (UNII: V5RS026Q0H) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) PEG-100 STEARATE (UNII: YD01N1999R) MICA (UNII: V8A1AW0880) STEARETH-21 (UNII: 53J3F32P58) STEARETH-2 (UNII: V56DFE46J5) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CAPRYLYL GLYCOL (UNII: 00YIU5438U) GREEN TEA LEAF (UNII: W2ZU1RY8B0) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PHLEBODIUM AUREUM LEAF (UNII: BG58BW230G) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) HEXYLENE GLYCOL (UNII: KEH0A3F75J) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) CETEARETH-25 (UNII: 8FA93U5T67) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) LINALOOL, (+/-)- (UNII: D81QY6I88E) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) HYALURONATE SODIUM (UNII: YSE9PPT4TH) DIPROPYLENE GLYCOL DIBENZOATE (UNII: 6OA5ZDY41O) PROPANEDIOL (UNII: 5965N8W85T) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) PHENOXYETHANOL (UNII: HIE492ZZ3T) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) DISODIUM ETHYLENE DICOCAMIDE PEG-15 DISULFATE (UNII: QI9A6U005W) PPG-15 STEARYL ETHER BENZOATE (UNII: 80D2J6361M) PHENYLPROPANOL (UNII: 0F897O3O4M) PANTHENOL (UNII: WV9CM0O67Z) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82141-2339-1 1 in 1 CARTON 10/01/2022 1 40 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 10/01/2022 TOTY ILUMINA CC CREAM 3N
titanium dioxide, zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82141-2333 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 9 g in 100 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 6.3 g in 100 g Inactive Ingredients Ingredient Name Strength 1,3-BUTYLENE GLYCOL COCOATE (UNII: B2JUC5N9EH) BUTYL METHACRYLATE/METHYL METHACRYLATE/METHACRYLIC ACID/STYRENE CROSSPOLYMER (UNII: V5RS026Q0H) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CAPRYLYL GLYCOL (UNII: 00YIU5438U) GREEN TEA LEAF (UNII: W2ZU1RY8B0) STEARALKONIUM HECTORITE (UNII: OLX698AH5P) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PHLEBODIUM AUREUM LEAF (UNII: BG58BW230G) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) COCO GLUCOSIDE (UNII: ICS790225B) WATER (UNII: 059QF0KO0R) HEXYLENE GLYCOL (UNII: KEH0A3F75J) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) CETEARETH-25 (UNII: 8FA93U5T67) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) LINALOOL, (+/-)- (UNII: D81QY6I88E) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) HYALURONATE SODIUM (UNII: YSE9PPT4TH) DICAPRYLYL CARBONATE (UNII: 609A3V1SUA) DIPROPYLENE GLYCOL DIBENZOATE (UNII: 6OA5ZDY41O) PROPANEDIOL (UNII: 5965N8W85T) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) PHENOXYETHANOL (UNII: HIE492ZZ3T) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) DISODIUM ETHYLENE DICOCAMIDE PEG-15 DISULFATE (UNII: QI9A6U005W) PPG-15 STEARYL ETHER BENZOATE (UNII: 80D2J6361M) PHENYLPROPANOL (UNII: 0F897O3O4M) MYRISTYL ALCOHOL (UNII: V42034O9PU) MYRISTYL GLUCOSIDE (UNII: 6AK28695LF) PANTHENOL (UNII: WV9CM0O67Z) PROPYLENE CARBONATE (UNII: 8D08K3S51E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82141-2333-1 1 in 1 CARTON 10/01/2022 1 40 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 10/01/2022 TOTY ILUMINA CC CREAM 4W
titanium dioxide, zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82141-2337 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 9 g in 100 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 6.3 g in 100 g Inactive Ingredients Ingredient Name Strength BUTYL METHACRYLATE/METHYL METHACRYLATE/METHACRYLIC ACID/STYRENE CROSSPOLYMER (UNII: V5RS026Q0H) 1,3-BUTYLENE GLYCOL COCOATE (UNII: B2JUC5N9EH) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) PEG-100 STEARATE (UNII: YD01N1999R) MICA (UNII: V8A1AW0880) STEARETH-21 (UNII: 53J3F32P58) STEARETH-2 (UNII: V56DFE46J5) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CAPRYLYL GLYCOL (UNII: 00YIU5438U) GREEN TEA LEAF (UNII: W2ZU1RY8B0) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PHLEBODIUM AUREUM LEAF (UNII: BG58BW230G) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) HEXYLENE GLYCOL (UNII: KEH0A3F75J) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) CETEARETH-25 (UNII: 8FA93U5T67) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) LINALOOL, (+/-)- (UNII: D81QY6I88E) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) HYALURONATE SODIUM (UNII: YSE9PPT4TH) DIPROPYLENE GLYCOL DIBENZOATE (UNII: 6OA5ZDY41O) PROPANEDIOL (UNII: 5965N8W85T) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) PHENOXYETHANOL (UNII: HIE492ZZ3T) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) DISODIUM ETHYLENE DICOCAMIDE PEG-15 DISULFATE (UNII: QI9A6U005W) PPG-15 STEARYL ETHER BENZOATE (UNII: 80D2J6361M) PHENYLPROPANOL (UNII: 0F897O3O4M) PANTHENOL (UNII: WV9CM0O67Z) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82141-2337-1 1 in 1 CARTON 10/01/2022 1 40 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 10/01/2022 TOTY ILUMINA CC CREAM 2C
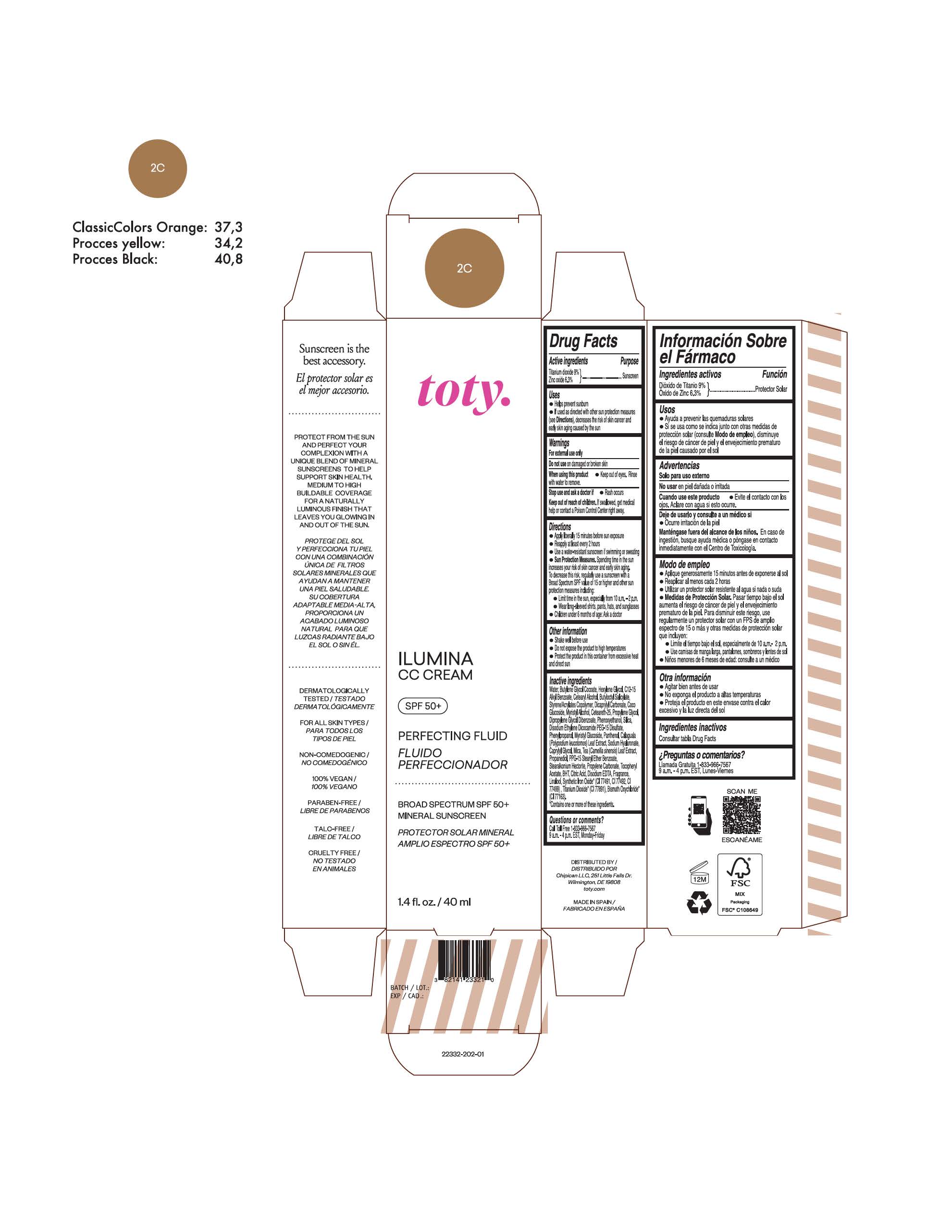
titanium dioxide, zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82141-2332 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 9 g in 100 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 6.3 g in 100 g Inactive Ingredients Ingredient Name Strength 1,3-BUTYLENE GLYCOL COCOATE (UNII: B2JUC5N9EH) BUTYL METHACRYLATE/METHYL METHACRYLATE/METHACRYLIC ACID/STYRENE CROSSPOLYMER (UNII: V5RS026Q0H) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CAPRYLYL GLYCOL (UNII: 00YIU5438U) GREEN TEA LEAF (UNII: W2ZU1RY8B0) STEARALKONIUM HECTORITE (UNII: OLX698AH5P) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PHLEBODIUM AUREUM LEAF (UNII: BG58BW230G) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) COCO GLUCOSIDE (UNII: ICS790225B) WATER (UNII: 059QF0KO0R) HEXYLENE GLYCOL (UNII: KEH0A3F75J) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) CETEARETH-25 (UNII: 8FA93U5T67) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) LINALOOL, (+/-)- (UNII: D81QY6I88E) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) HYALURONATE SODIUM (UNII: YSE9PPT4TH) DICAPRYLYL CARBONATE (UNII: 609A3V1SUA) DIPROPYLENE GLYCOL DIBENZOATE (UNII: 6OA5ZDY41O) PROPANEDIOL (UNII: 5965N8W85T) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) PHENOXYETHANOL (UNII: HIE492ZZ3T) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) DISODIUM ETHYLENE DICOCAMIDE PEG-15 DISULFATE (UNII: QI9A6U005W) PPG-15 STEARYL ETHER BENZOATE (UNII: 80D2J6361M) PHENYLPROPANOL (UNII: 0F897O3O4M) MYRISTYL ALCOHOL (UNII: V42034O9PU) MYRISTYL GLUCOSIDE (UNII: 6AK28695LF) PANTHENOL (UNII: WV9CM0O67Z) PROPYLENE CARBONATE (UNII: 8D08K3S51E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82141-2332-1 1 in 1 CARTON 10/01/2022 1 40 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 10/01/2022 TOTY ILUMINA CC CREAM 2W
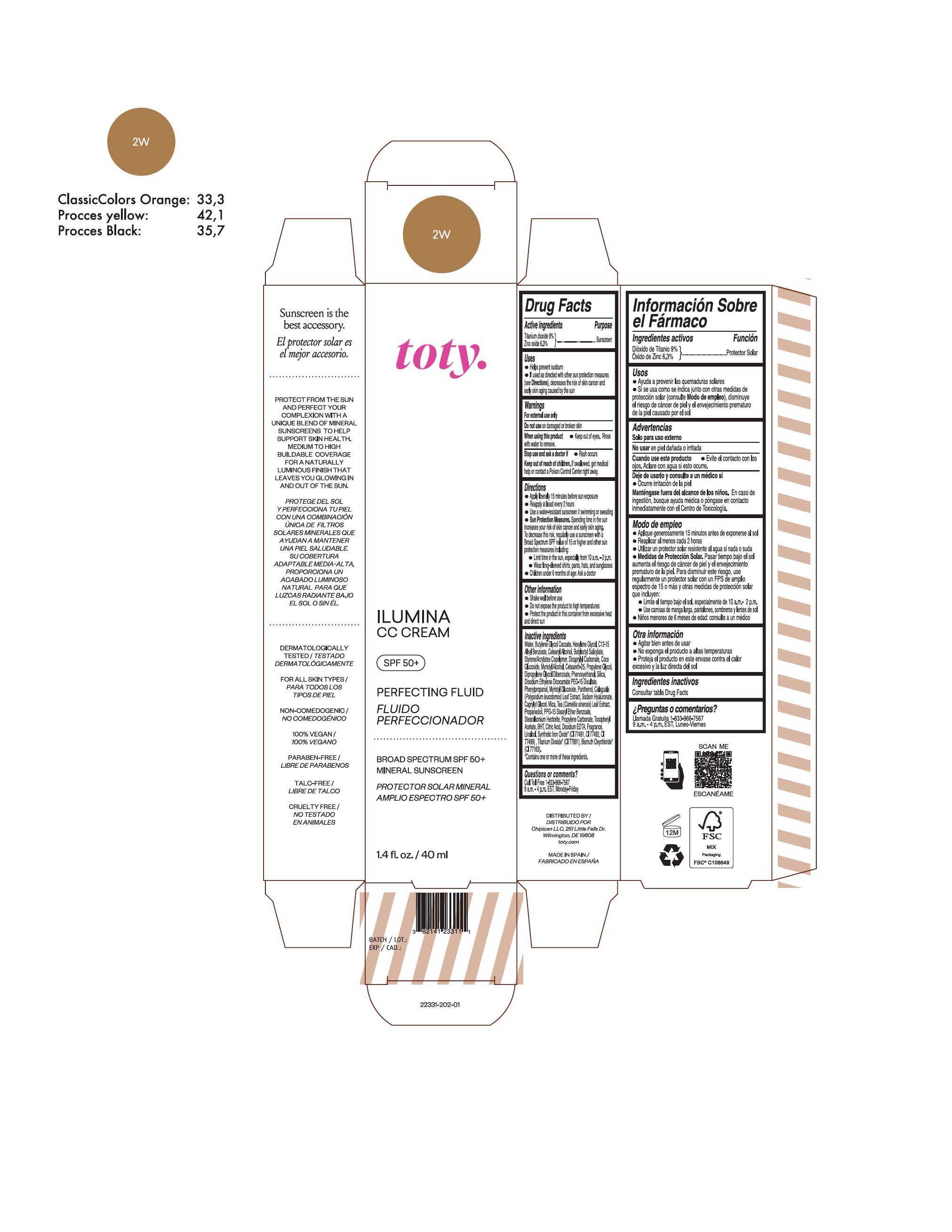
titanium dioxide, zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82141-2331 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 9 g in 100 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 6.3 g in 100 g Inactive Ingredients Ingredient Name Strength BUTYL METHACRYLATE/METHYL METHACRYLATE/METHACRYLIC ACID/STYRENE CROSSPOLYMER (UNII: V5RS026Q0H) 1,3-BUTYLENE GLYCOL COCOATE (UNII: B2JUC5N9EH) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CAPRYLYL GLYCOL (UNII: 00YIU5438U) GREEN TEA LEAF (UNII: W2ZU1RY8B0) STEARALKONIUM HECTORITE (UNII: OLX698AH5P) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PHLEBODIUM AUREUM LEAF (UNII: BG58BW230G) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) COCO GLUCOSIDE (UNII: ICS790225B) WATER (UNII: 059QF0KO0R) HEXYLENE GLYCOL (UNII: KEH0A3F75J) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) CETEARETH-25 (UNII: 8FA93U5T67) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) LINALOOL, (+/-)- (UNII: D81QY6I88E) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) HYALURONATE SODIUM (UNII: YSE9PPT4TH) DICAPRYLYL CARBONATE (UNII: 609A3V1SUA) DIPROPYLENE GLYCOL DIBENZOATE (UNII: 6OA5ZDY41O) PROPANEDIOL (UNII: 5965N8W85T) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) PHENOXYETHANOL (UNII: HIE492ZZ3T) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) DISODIUM ETHYLENE DICOCAMIDE PEG-15 DISULFATE (UNII: QI9A6U005W) PPG-15 STEARYL ETHER BENZOATE (UNII: 80D2J6361M) PHENYLPROPANOL (UNII: 0F897O3O4M) MYRISTYL ALCOHOL (UNII: V42034O9PU) MYRISTYL GLUCOSIDE (UNII: 6AK28695LF) PANTHENOL (UNII: WV9CM0O67Z) PROPYLENE CARBONATE (UNII: 8D08K3S51E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82141-2331-1 1 in 1 CARTON 10/01/2022 1 40 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 10/01/2022 Labeler - Chipican LLC (118132015) Establishment Name Address ID/FEI Business Operations Industrial Farmaceutica Cantabria SA 470471158 manufacture(82141-2327, 82141-2328, 82141-2329, 82141-2330, 82141-2331, 82141-2332, 82141-2333, 82141-2334, 82141-2335, 82141-2336, 82141-2337, 82141-2338, 82141-2339, 82141-2340, 82141-2341) , pack(82141-2327, 82141-2328, 82141-2329, 82141-2330, 82141-2331, 82141-2332, 82141-2333, 82141-2334, 82141-2335, 82141-2336, 82141-2337, 82141-2338, 82141-2339, 82141-2340, 82141-2341) , label(82141-2327, 82141-2328, 82141-2329, 82141-2330, 82141-2331, 82141-2332, 82141-2333, 82141-2334, 82141-2335, 82141-2336, 82141-2337, 82141-2338, 82141-2339, 82141-2340, 82141-2341)