Uses
- Helps prevent sunburn
- If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
Warnings
For external use only
Do not use on damaged or broken skin
When using this product Keep out of eyes. Rinse
with water to remove.
Stop use and ask a doctor if Rash occurs
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
- Apply liberally 15 minutes behore sun exposure
- Reapply at least every 2 hours
- Use a water-resistant sunscreen if swimmimg or sweating
- Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- Limit time in the sun, especially from 10 a.m. - 2 p.m
- Wear long-sleeved shirts, pants, hats, and sunglasses
- Children under 6 months of age: Ask a doctor.
Other Information
- Shake well before use
- Do not expose the product to high temperatures
- Protect the product in this container from excessive heat and direct sun
Inactive Ingredients
NDCs 82141-2327-1, 82141-2328-1
Water, Butylene Glycol Cocoate,Hexylene Glycol, C12-15
Alkyl Benzoate, Cetearyl Alcohol, Butyloctyl Salicylate,
Styrene/Acrylates Copolymer, Dicaprylyl Carbonate, Coco
Glucoside, Myristyl Alcohol, Ceteareth-25, Propylene Glycol,
Dipropylene Glycol Dibenzoate, Phenoxyethanol, Silica,
Disodium Ethylene Dicocamide PEG-15 Disulfate,
Phenylpropanol, Myristyl Glucoside, Panthenol, Calaguala
( Polypodium leucotomos) Leaf Extract, Sodium Hyaluronate,
Caprylyl Glycol, Tea ( Camellia sinensis) Leaf Extract,
Propanediol, PPG-15 Stearyl Ether Benzoate,
Stearalkonium Hectorite, Propylene Carbonate, Tocopheryl
Acetate, BHT, Citric Acid, Disodium EDTA, Fragrance,
Linalool, Synthetic Iron Oxide* (CI 77491, CI 77492, CI
77499), Titanium Dioxide* (CI 77891), Bismuth Oxychloride*
(CI 77163).
* Contains one or more of these ingredients.
NDCs 82141-2329-1, 82141-2330-1, 82141-2331-1, 82141-2332-1, 82141-2333-1
82141-2334-1, 82141-2336-1 & 82141-2338-1
Water, Butylene Glycol Cocoate,Hexylene Glycol, C12-15
Alkyl Benzoate, Cetearyl Alcohol, Butyloctyl Salicylate,
Styrene/Acrylates Copolymer, Dicaprylyl Carbonate, Coco
Glucoside, Myristyl Alcohol, Ceteareth-25, Propylene Glycol,
Dipropylene Glycol Dibenzoate, Phenoxyethanol, Silica,
Disodium Ethylene Dicocamide PEG-15 Disulfate,
Phenylpropanol, Myristyl Glucoside, Panthenol, Calaguala
( Polypodium leucotomos) Leaf Extract, Sodium Hyaluronate,
Caprylyl Glycol, Mica, Tea ( Camellia sinensis) Leaf Extract,
Propanediol, PPG-15 Stearyl Ether Benzoate,
Stearalkonium Hectorite, Propylene Carbonate, Tocopheryl
Acetate, BHT, Citric Acid, Disodium EDTA, Fragrance,
Linalool, Synthetic Iron Oxide* (CI 77491, CI 77492, CI
77499), Titanium Dioxide* (CI 77891), Bismuth Oxychloride*
(CI 77163).
* Contains one or more of these ingredients.
NDCs 82141-2335-1
Water, Butylene Glycol Cocoate,Hexylene Glycol, C12-15
Alkyl Benzoate, Cetearyl Alcohol, Butyloctyl Salicylate,
Styrene/Acrylates Copolymer, Dicaprylyl Carbonate, Coco
Glucoside, Myristyl Alcohol, Ceteareth-25, Propylene Glycol,
Dipropylene Glycol Dibenzoate, Phenoxyethanol, Silica,
Disodium Ethylene Dicocamide PEG-15 Disulfate,
Phenylpropanol, Myristyl Glucoside, Panthenol, Calaguala
( Polypodium leucotomos) Leaf Extract, Sodium Hyaluronate,
Caprylyl Glycol, Mica, Tea ( Camellia sinensis) Leaf Extract,
Propanediol, PPG-15 Stearyl Ether Benzoate,
Stearalkonium Hectorite, Propylene Carbonate, Tocopheryl
Acetate, BHT, Citric Acid, Disodium EDTA,
Triethoxycaprylylsilane, Fragrance, Linalool, Synthetic Iron
Oxide* (CI 77491, CI 77492, CI 77499), Titanium Dioxide*
(CI 77891), Bismuth Oxychloride* (CI 77163).
* Contains one or more of these ingredients.
NDCs 82141-2337-1 &82141-2339-1
Water, Butylene Glycol Cocoate,Hexylene Glycol, C12-15
Alkyl Benzoate, Butyloctyl Salicylate, Styrene/Acrylates
Copolymer, Ceteareth-25, Glyceril Stearate, PEG-100
Stearate, Propylene Glycol, Dipropylene Glycol Dibenzoate,
Silica, Phenoxyethanol, Disodium Ethylene Dicocamide
PEG-15 Disulfate, Phenylpropanol, Panthenol, Calaguala
( Polypodium leucotomos) Leaf Extract, Sodium Hyaluronate,
Caprylyl Glycol, Mica, Tea ( Camellia sinensis) Leaf Extract,
Propanediol, PPG-15 Stearyl Ether Benzoate, Tocopheryl
Acetate, Steareth-21, Steareth-2, Triethoxycaprylylsilane,
BHT, Citric Acid, Disodium EDTA, Fragrance, Linalool,
Synthetic Iron Oxide* (CI 77491, CI 77492, CI 77499).
* Contains one or more of these ingredients.
NDCs 82141-2340-1 & 82141-2341-1
Water, Butylene Glycol Cocoate,Hexylene Glycol, C12-15
Alkyl Benzoate, Butyloctyl Salicylate, Styrene/Acrylates
Copolymer, Ceteareth-25, Propylene Glycol, Dipropylene
Glycol Dibenzoate, Silica, Phenoxyethanol, Phenylpropanol,
Disodium Ethylene Dicocamide PEG-15 Disulfate,
Panthenol, Calaguala ( Polypodium leucotomos) Leaf
Extract, Sodium Hyaluronate, Caprylyl Glycol, Mica, Tea
( Camellia sinensis) Leaf Extract, Propanediol, PPG-15
Stearyl Ether Benzoate, Tocopheryl Acetate, Glyceryl
Stearate, PEG-100 Stearate, Steareth-21, Steareth-2,
Triethoxycaprylylsilane, BHT, Citric Acid, Disodium EDTA,
Fragrance, Linalool, Synthetic Iron Oxide* (CI 77491, CI
77492, CI 77499).
* Contains one or more of these ingredients.
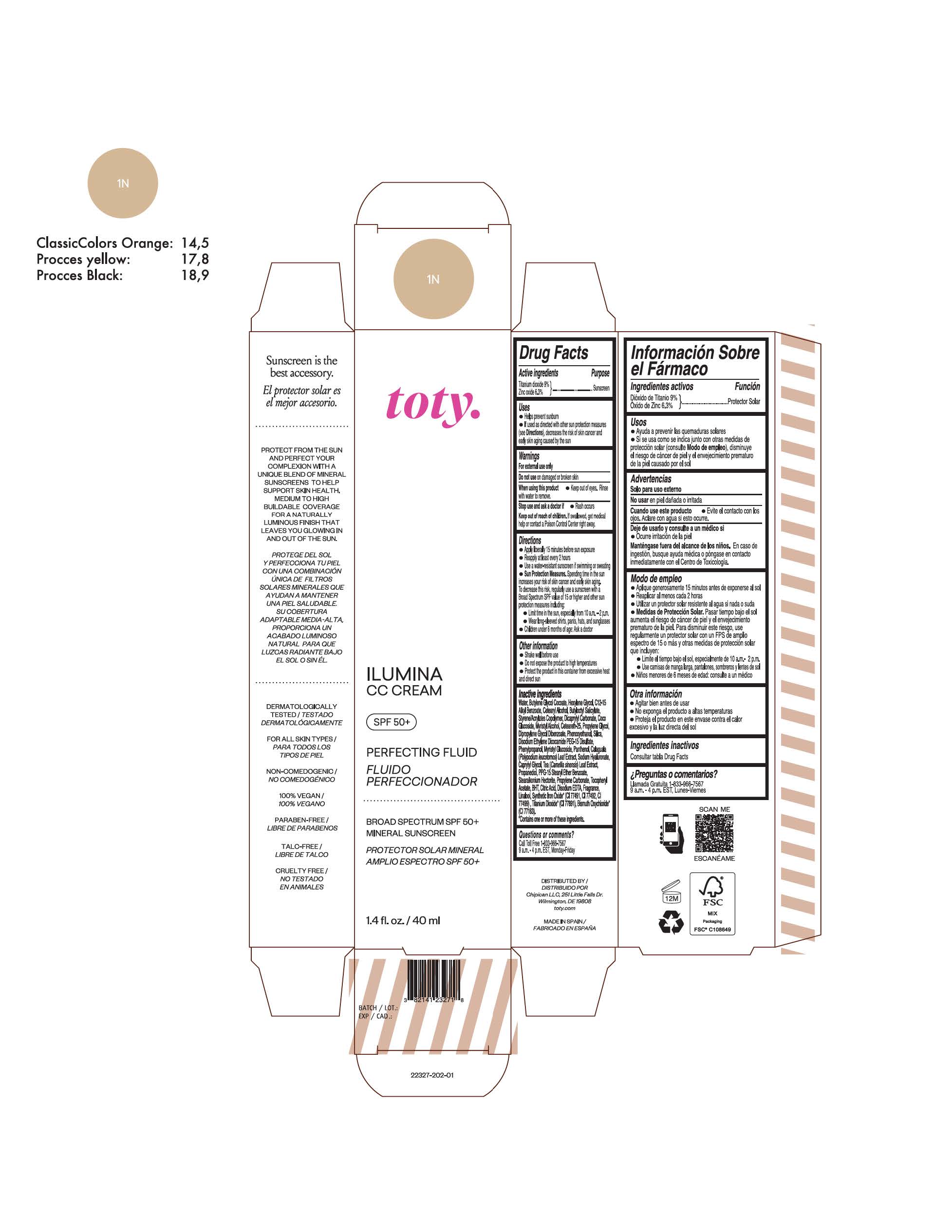
toty Ilumina CC Cream 1N
NDC 82141-2327-1 (toty Ilumina CC Cream 1N)
toty.
ILUMINA
CC CREAM
SPF 50+
Perfecting Fluid
Fluido
Perfeccionador
.......................................................
BROAD SPECTRUM SPF 50+
MINERAL SUNSCREEN
PROTECTOR SOLAR MINERAL
AMPLIO ESPECTRO SPF 50+
1.4 fl. oz./ 40 ml
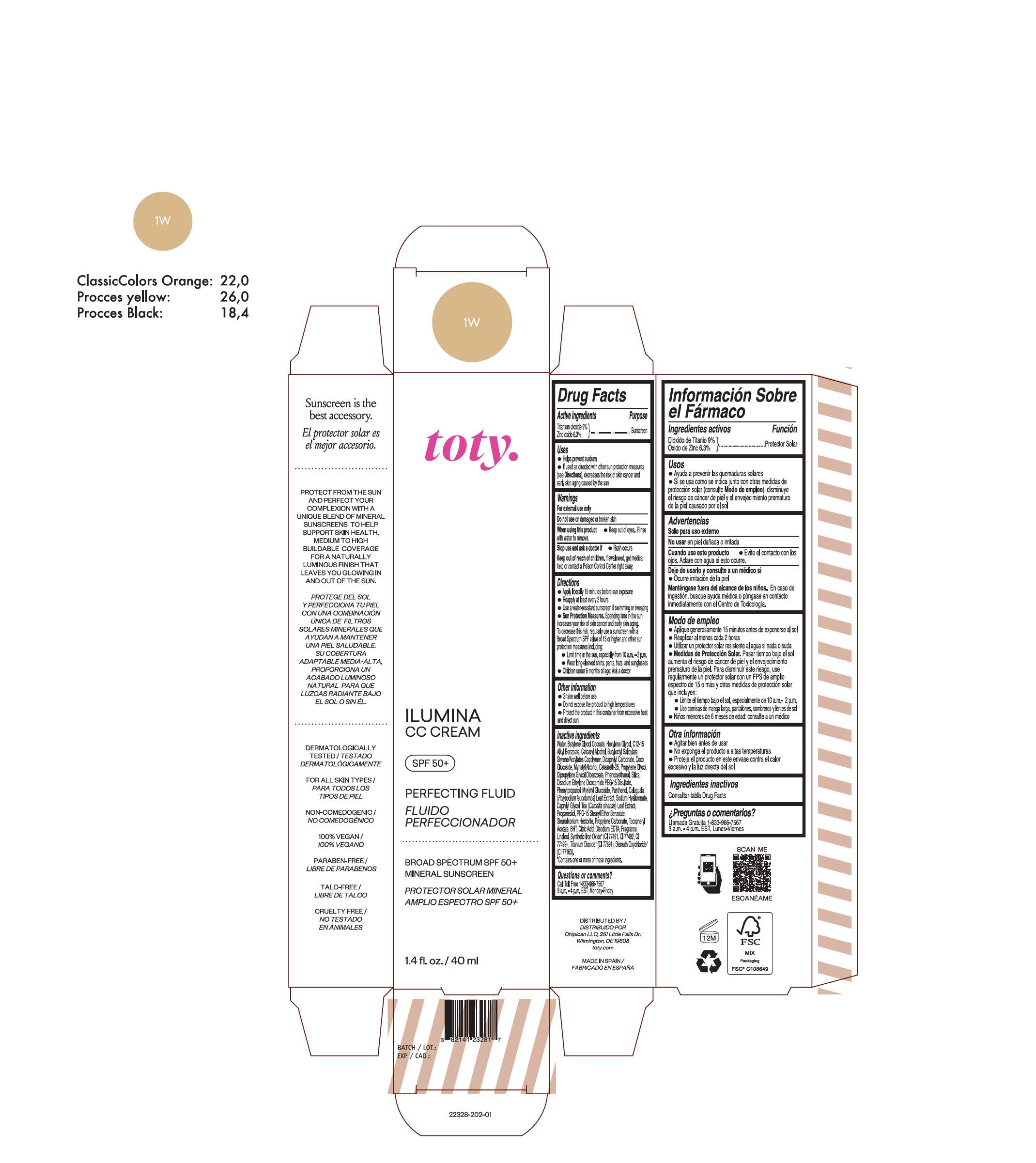
toty Ilumina CC Cream 1W
NDC 82141-2328-1 (toty Ilumina CC Cream 1W)
toty.
ILUMINA
CC CREAM
SPF 50+
Perfecting Fluid
Fluido
Perfeccionador
.......................................................
BROAD SPECTRUM SPF 50+
MINERAL SUNSCREEN
PROTECTOR SOLAR MINERAL
AMPLIO ESPECTRO SPF 50+
1.4 fl. oz./ 40 ml
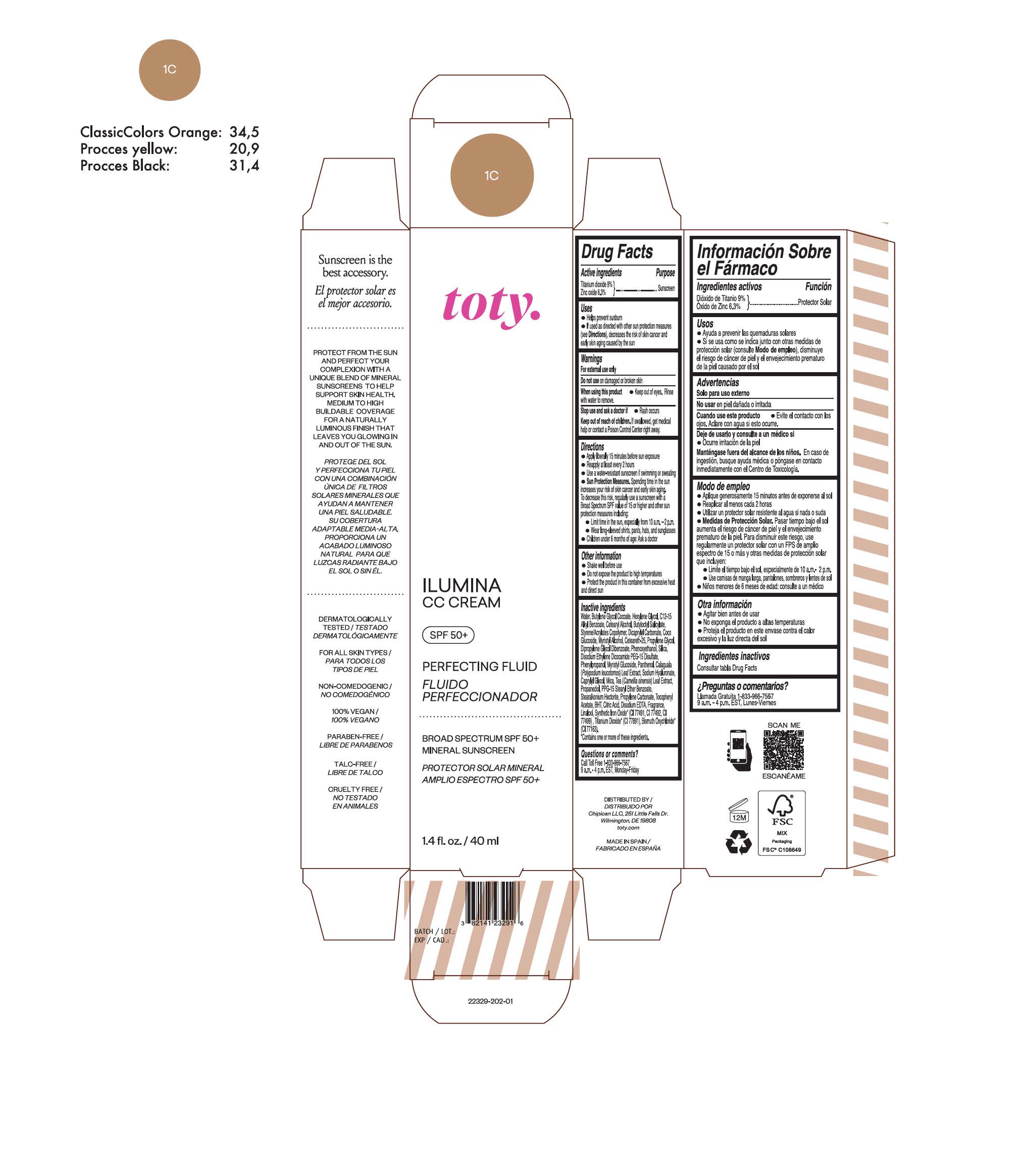
toty Ilumina CC Cream 1C
NDC 82141-2329-1 (toty Ilumina CC Cream 1C)
toty.
ILUMINA
CC CREAM
SPF 50+
Perfecting Fluid
Fluido
Perfeccionador
.......................................................
BROAD SPECTRUM SPF 50+
MINERAL SUNSCREEN
PROTECTOR SOLAR MINERAL
AMPLIO ESPECTRO SPF 50+
1.4 fl. oz./ 40 ml
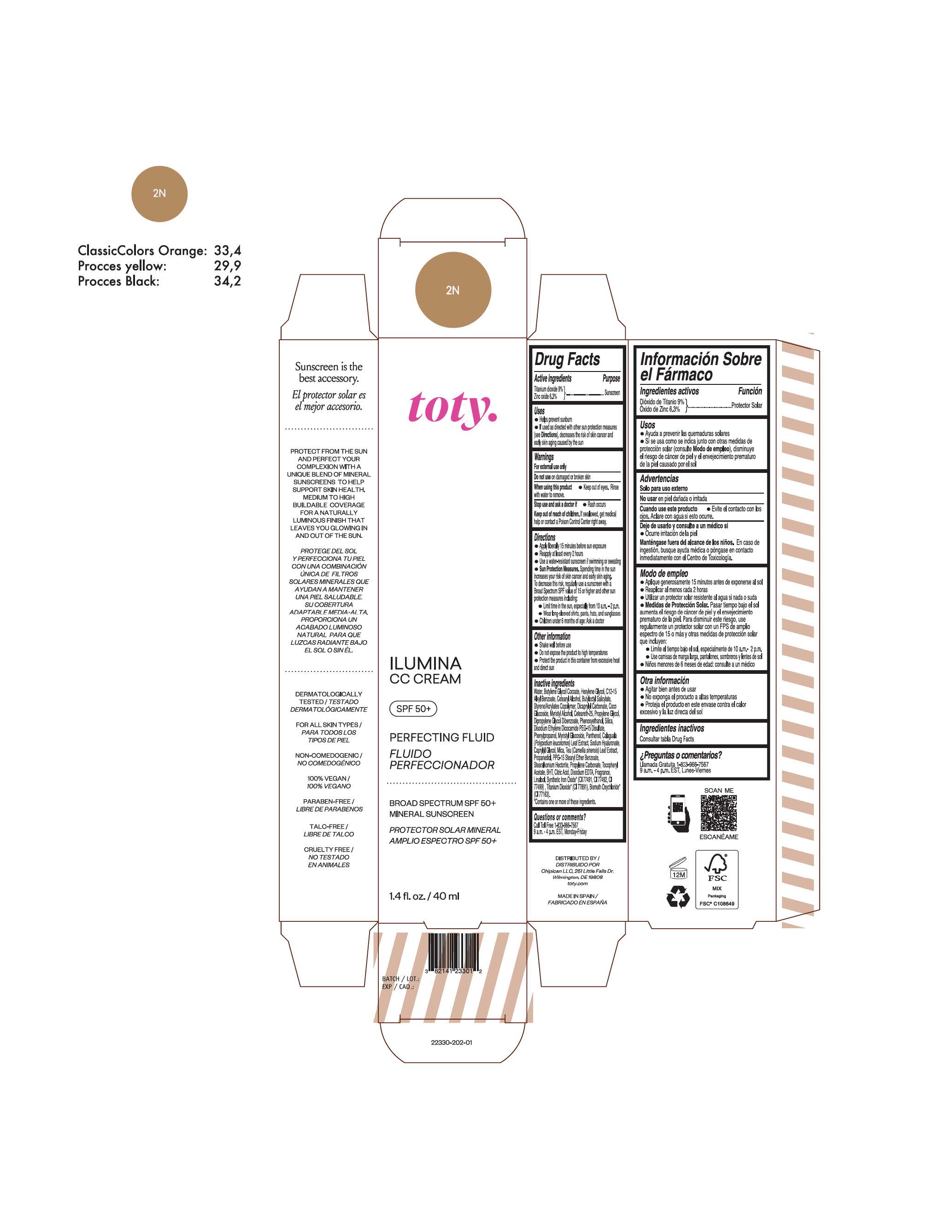
toty Ilumina CC Cream 2N
NDC 82141-2330-1 (toty Ilumina CC Cream 2N)
toty.
ILUMINA
CC CREAM
SPF 50+
Perfecting Fluid
Fluido
Perfeccionador
.......................................................
BROAD SPECTRUM SPF 50+
MINERAL SUNSCREEN
PROTECTOR SOLAR MINERAL
AMPLIO ESPECTRO SPF 50+
1.4 fl. oz./ 40 ml
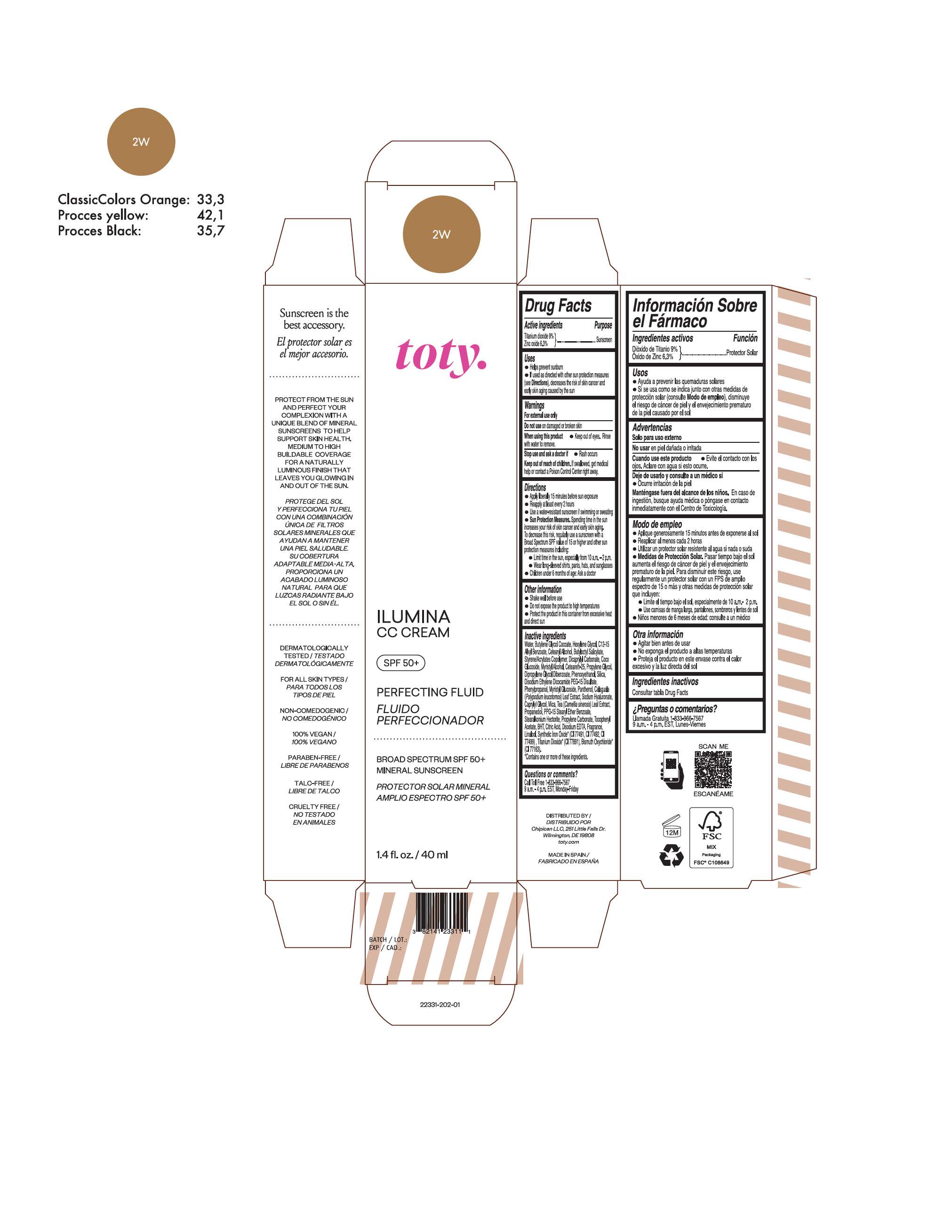
toty Ilumina CC Cream 2W
NDC 82141-2331-1 (toty Ilumina CC Cream 2W)
toty.
ILUMINA
CC CREAM
SPF 50+
Perfecting Fluid
Fluido
Perfeccionador
.......................................................
BROAD SPECTRUM SPF 50+
MINERAL SUNSCREEN
PROTECTOR SOLAR MINERAL
AMPLIO ESPECTRO SPF 50+
1.4 fl. oz./ 40 ml
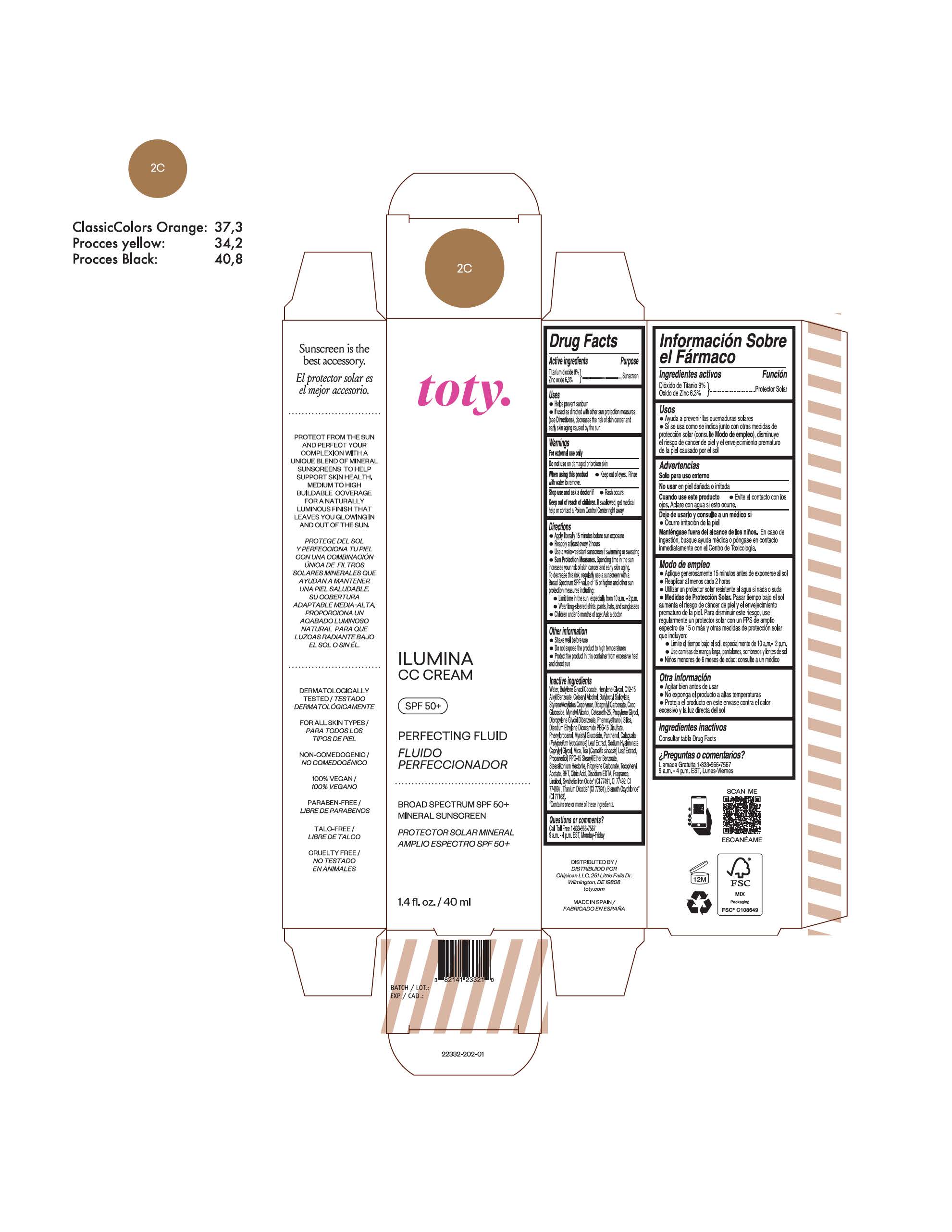
toty Ilumina CC Cream 2C
NDC 82141-2332-1 (toty Ilumina CC Cream 2C)
toty.
ILUMINA
CC CREAM
SPF 50+
Perfecting Fluid
Fluido
Perfeccionador
.......................................................
BROAD SPECTRUM SPF 50+
MINERAL SUNSCREEN
PROTECTOR SOLAR MINERAL
AMPLIO ESPECTRO SPF 50+
1.4 fl. oz./ 40 ml
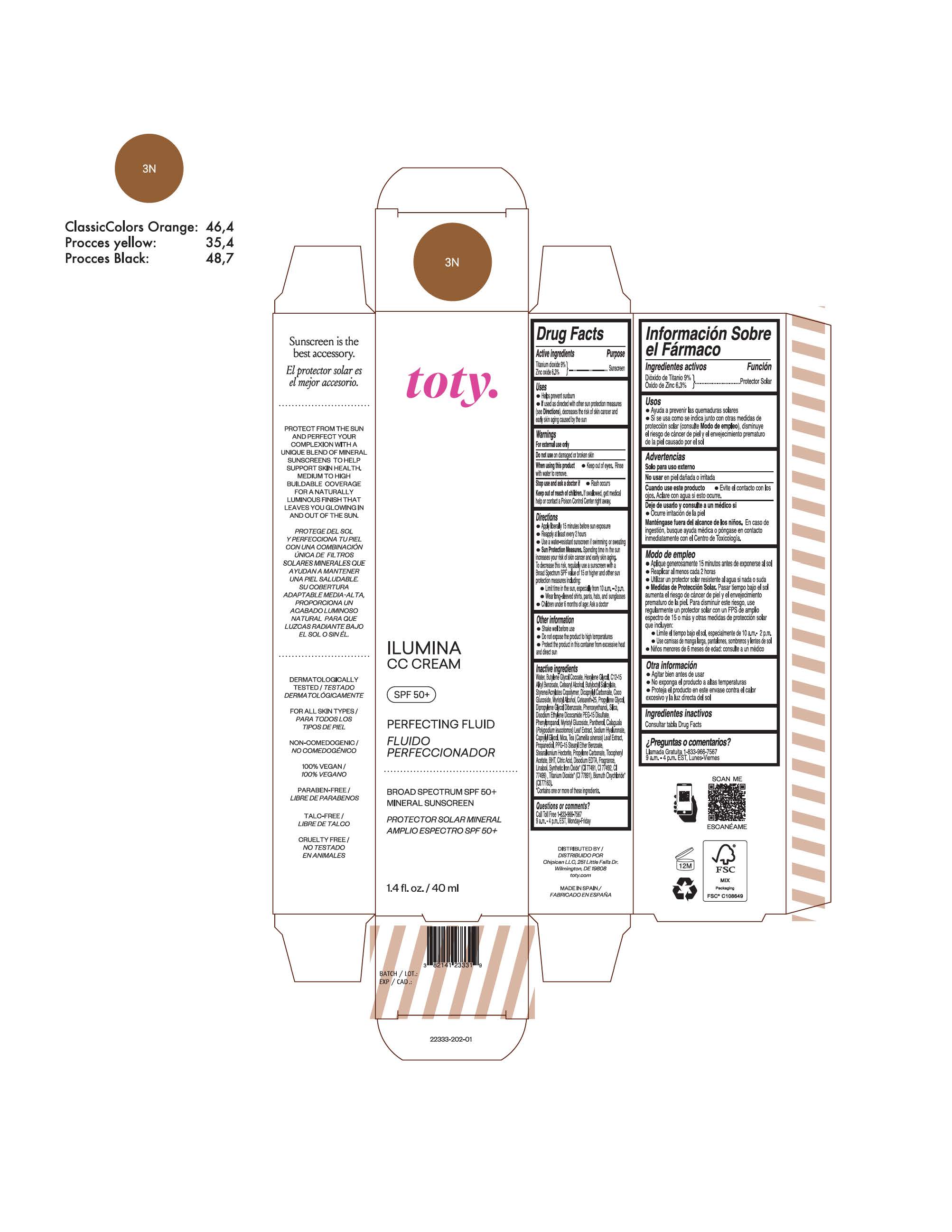
toty Ilumina CC Cream 3N
NDC 82141-2333-1 (toty Ilumina CC Cream 3N)
toty.
ILUMINA
CC CREAM
SPF 50+
Perfecting Fluid
Fluido
Perfeccionador
.......................................................
BROAD SPECTRUM SPF 50+
MINERAL SUNSCREEN
PROTECTOR SOLAR MINERAL
AMPLIO ESPECTRO SPF 50+
1.4 fl. oz./ 40 ml
toty Ilumina CC Cream 3W
NDC 82141-2334-1 (toty Ilumina CC Cream 3W)
toty.
ILUMINA
CC CREAM
SPF 50+
Perfecting Fluid
Fluido
Perfeccionador
.......................................................
BROAD SPECTRUM SPF 50+
MINERAL SUNSCREEN
PROTECTOR SOLAR MINERAL
AMPLIO ESPECTRO SPF 50+
1.4 fl. oz./ 40 ml
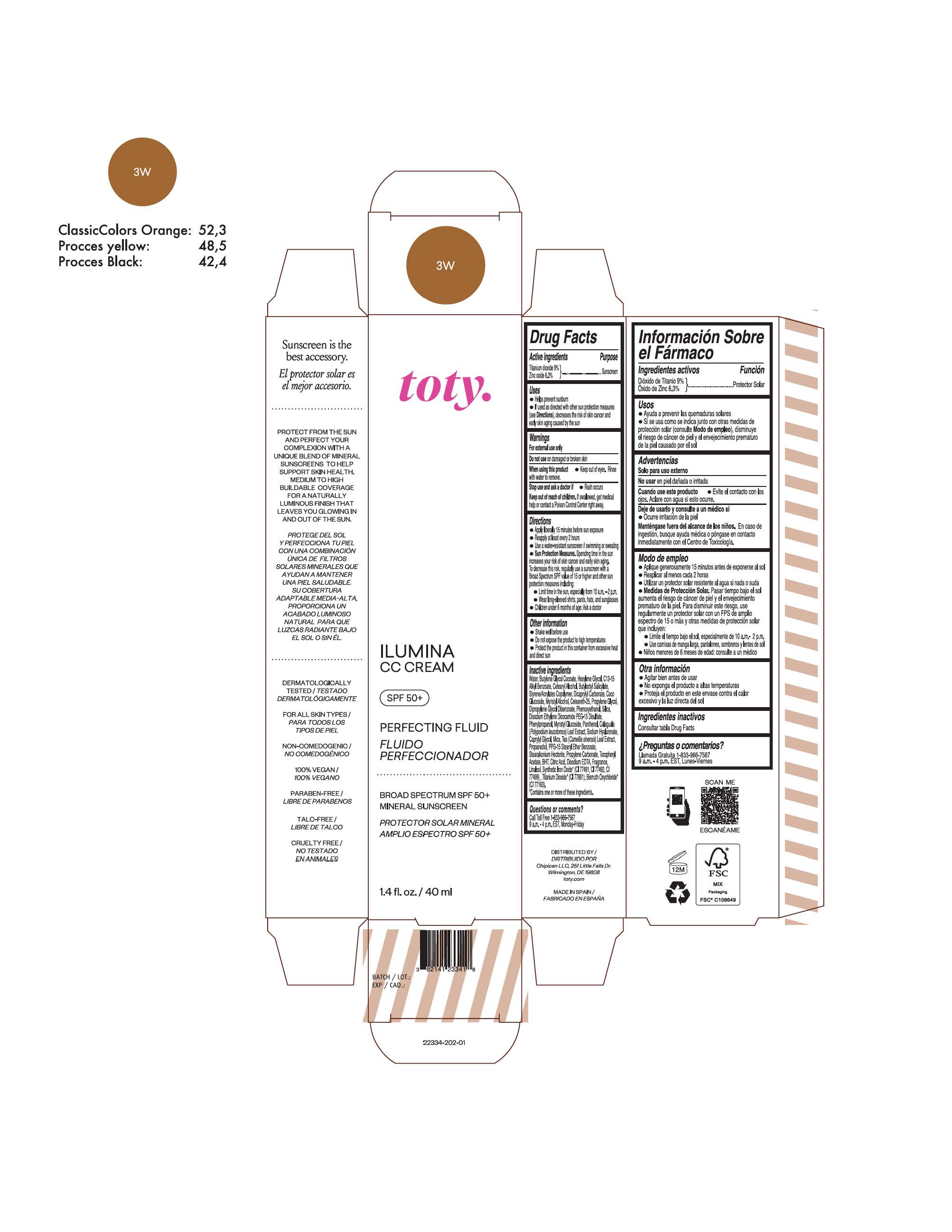
toty Ilumina CC Cream 3C
NDC 82141-2335-1 (toty Ilumina CC Cream 3C)
toty.
ILUMINA
CC CREAM
SPF 50+
Perfecting Fluid
Fluido
Perfeccionador
.......................................................
BROAD SPECTRUM SPF 50+
MINERAL SUNSCREEN
PROTECTOR SOLAR MINERAL
AMPLIO ESPECTRO SPF 50+
1.4 fl. oz./ 40 ml
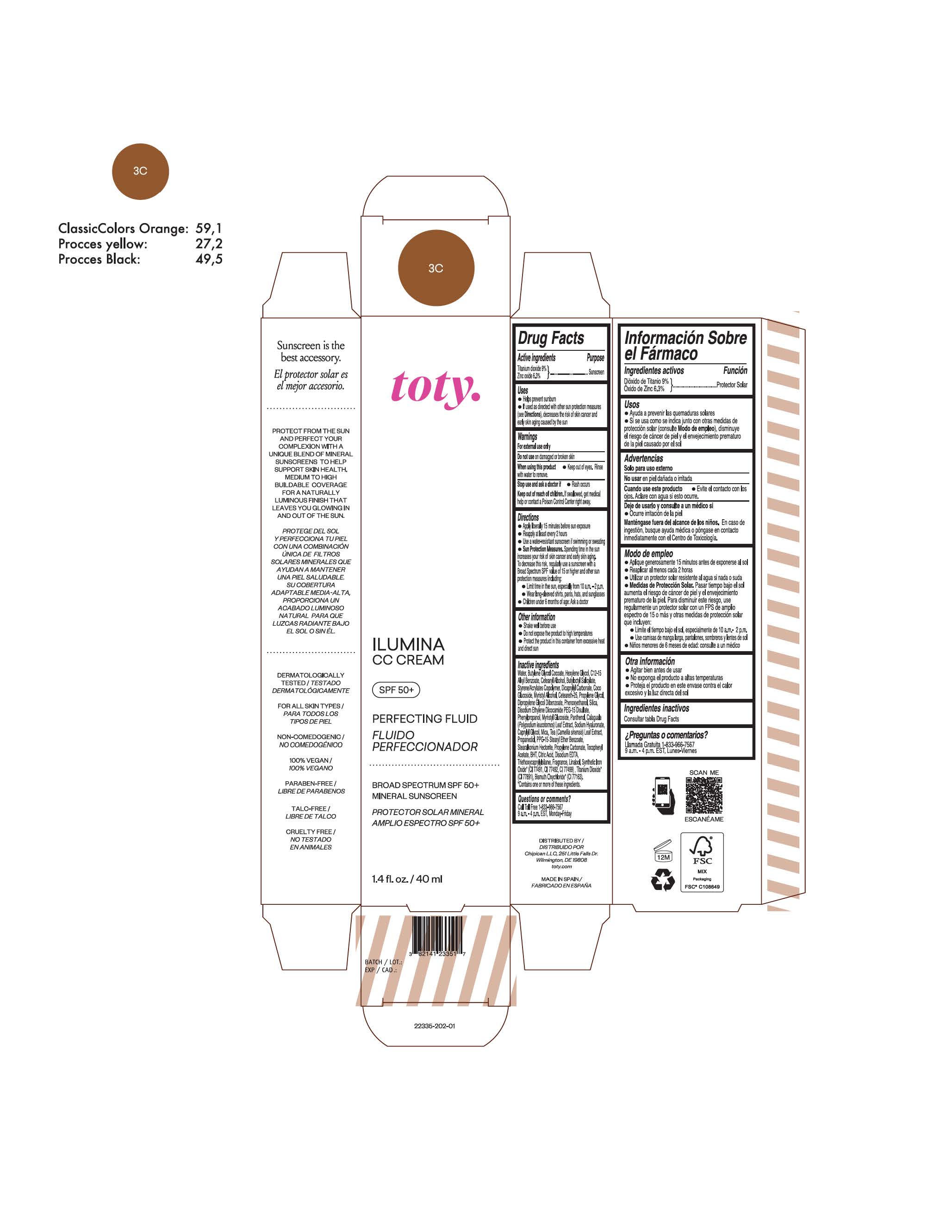
toty Ilumina CC Cream 4N
NDC 82141-2336-1 (toty Ilumina CC Cream 4N)
toty.
ILUMINA
CC CREAM
SPF 50+
Perfecting Fluid
Fluido
Perfeccionador
.......................................................
BROAD SPECTRUM SPF 50+
MINERAL SUNSCREEN
PROTECTOR SOLAR MINERAL
AMPLIO ESPECTRO SPF 50+
1.4 fl. oz./ 40 ml
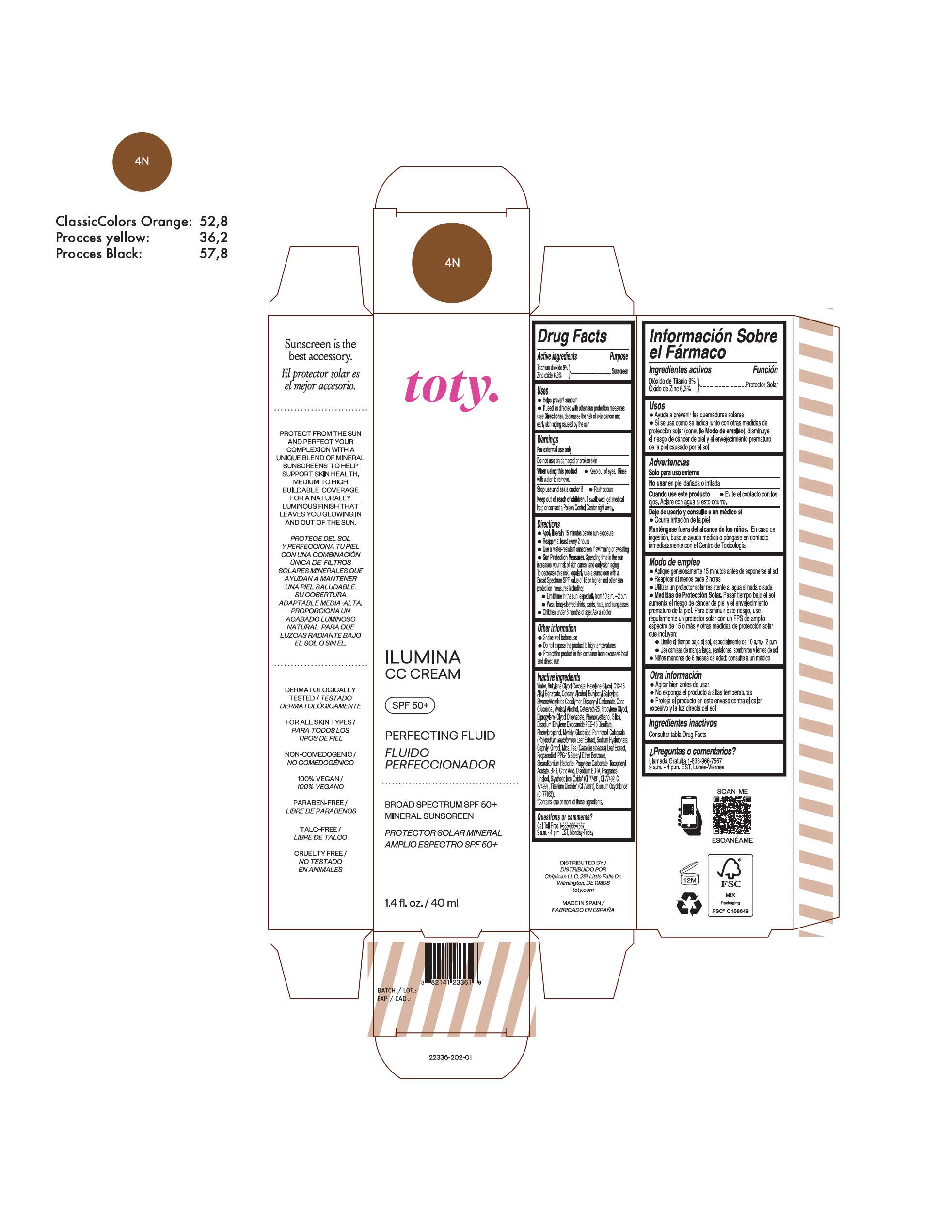
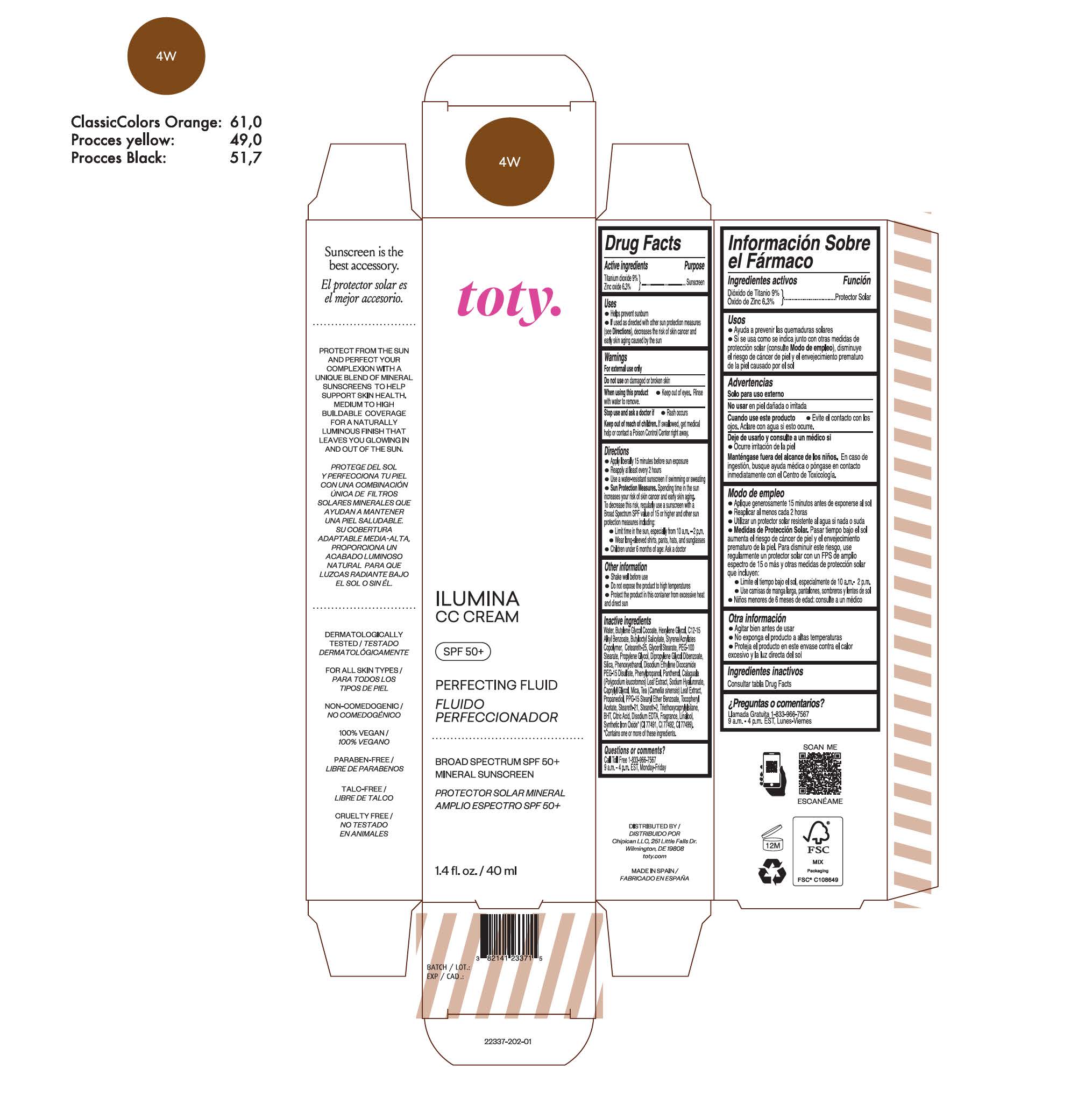
toty Ilumina CC Cream 4W
NDC 82141-2337-1 (toty Ilumina CC Cream 4W)
toty.
ILUMINA
CC CREAM
SPF 50+
Perfecting Fluid
Fluido
Perfeccionador
.......................................................
BROAD SPECTRUM SPF 50+
MINERAL SUNSCREEN
PROTECTOR SOLAR MINERAL
AMPLIO ESPECTRO SPF 50+
1.4 fl. oz./ 40 ml
toty Ilumina CC Cream 4C
NDC 82141-2338-1 (toty Ilumina CC Cream 4C)
toty.
ILUMINA
CC CREAM
SPF 50+
Perfecting Fluid
Fluido
Perfeccionador
.......................................................
BROAD SPECTRUM SPF 50+
MINERAL SUNSCREEN
PROTECTOR SOLAR MINERAL
AMPLIO ESPECTRO SPF 50+
1.4 fl. oz./ 40 ml
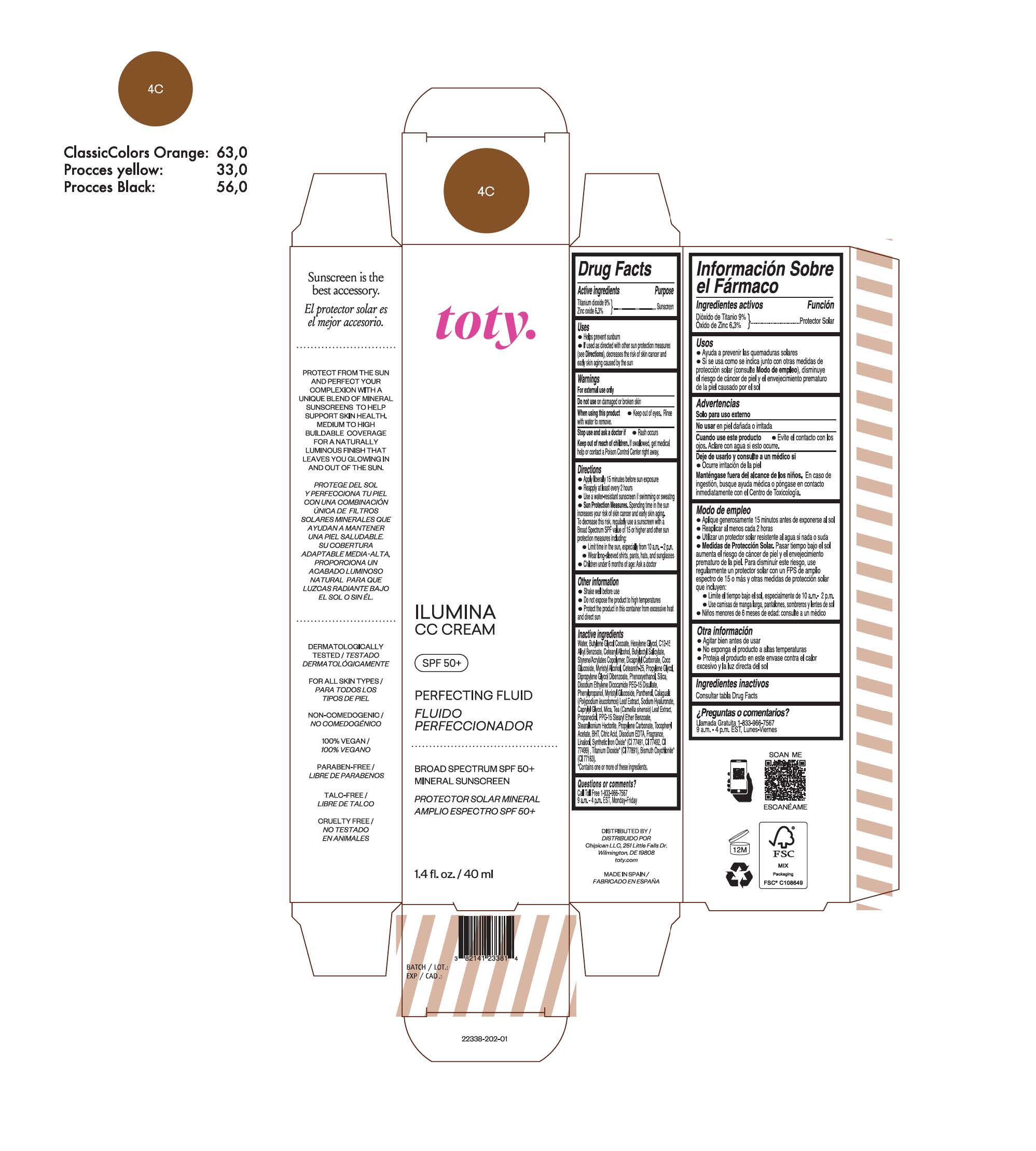
toty Ilumina CC Cream 5W
NDC 82141-2339-1 (toty Ilumina CC Cream 5W)
toty.
ILUMINA
CC CREAM
SPF 50+
Perfecting Fluid
Fluido
Perfeccionador
.......................................................
BROAD SPECTRUM SPF 50+
MINERAL SUNSCREEN
PROTECTOR SOLAR MINERAL
AMPLIO ESPECTRO SPF 50+
1.4 fl. oz./ 40 ml
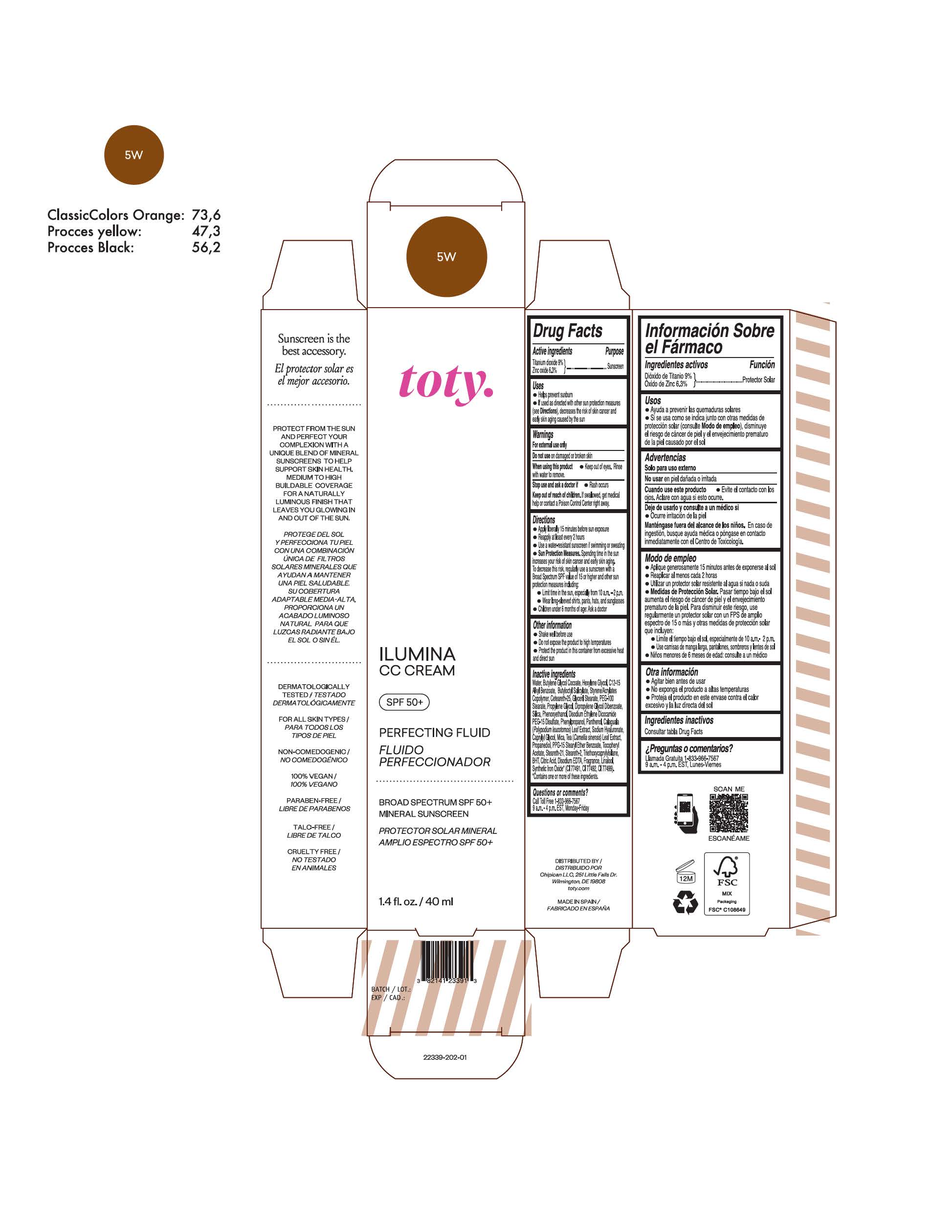
toty Ilumina CC Cream 5W1
NDC 82141-2340-1 (toty Ilumina CC Cream 5W1)
toty.
ILUMINA
CC CREAM
SPF 50+
Perfecting Fluid
Fluido
Perfeccionador
.......................................................
BROAD SPECTRUM SPF 50+
MINERAL SUNSCREEN
PROTECTOR SOLAR MINERAL
AMPLIO ESPECTRO SPF 50+
1.4 fl. oz./ 40 ml
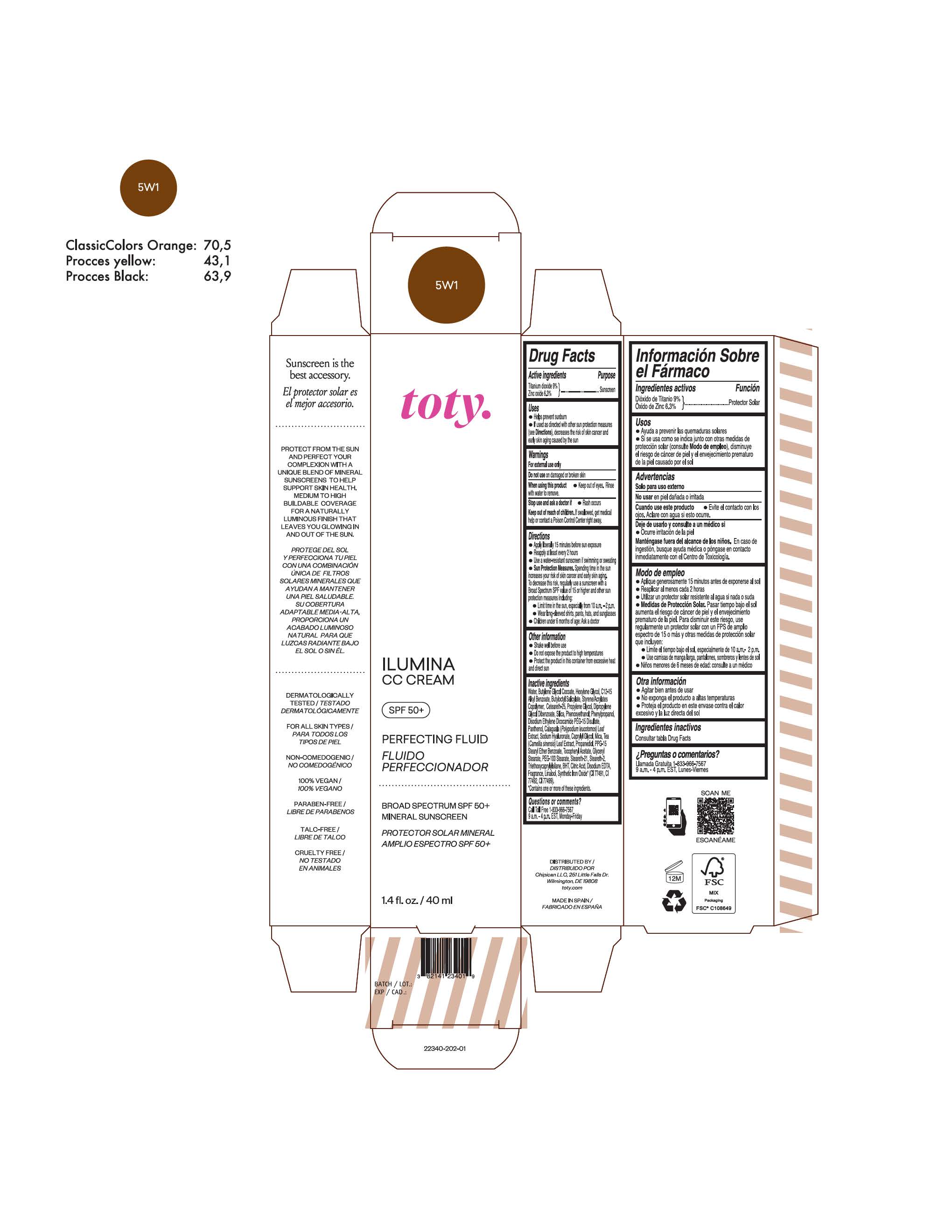
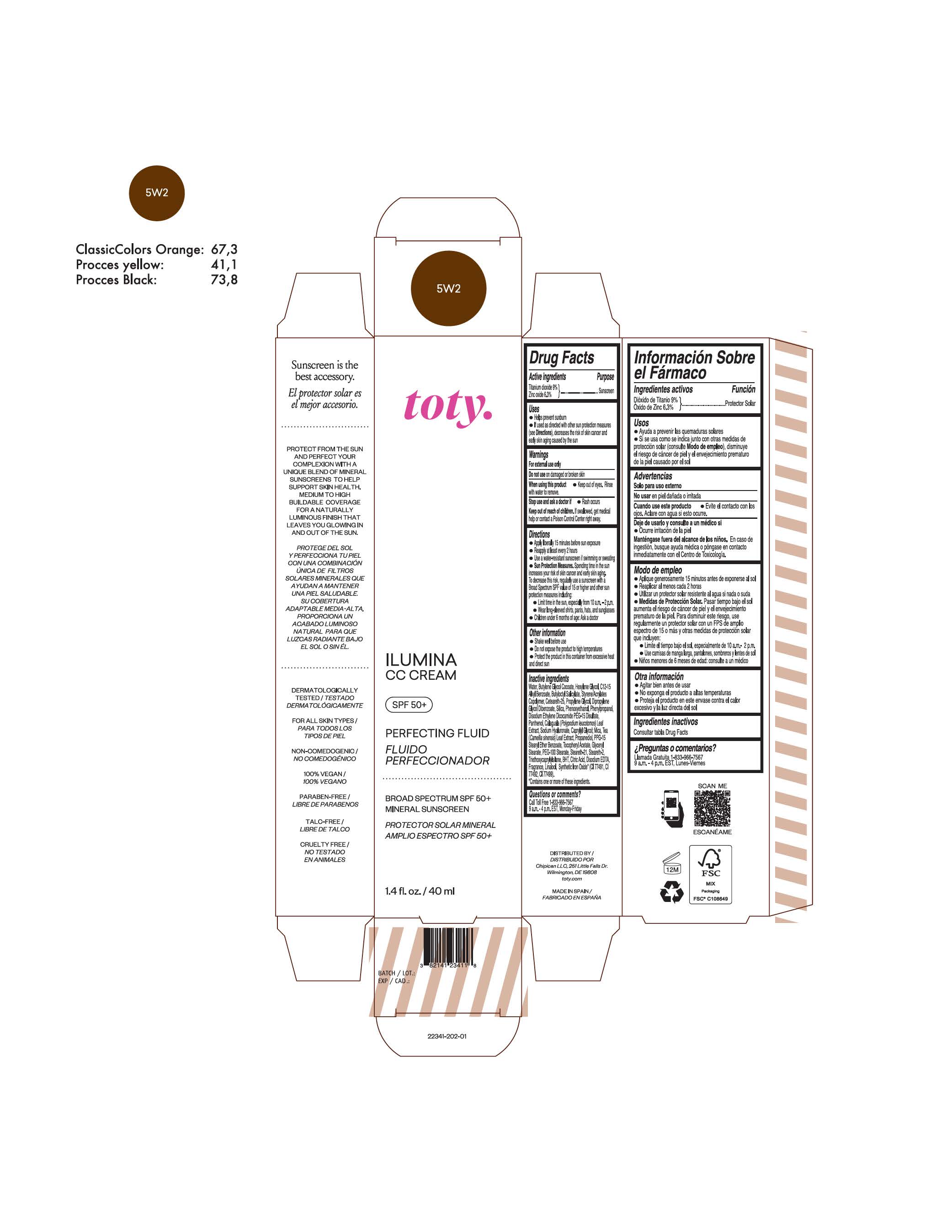
toty Ilumina CC Cream 5W2
NDC 82141-2341-1 (toty Ilumina CC Cream 5W2)
toty.
ILUMINA
CC CREAM
SPF 50+
Perfecting Fluid
Fluido
Perfeccionador
.......................................................
BROAD SPECTRUM SPF 50+
MINERAL SUNSCREEN
PROTECTOR SOLAR MINERAL
AMPLIO ESPECTRO SPF 50+
1.4 fl. oz./ 40 ml