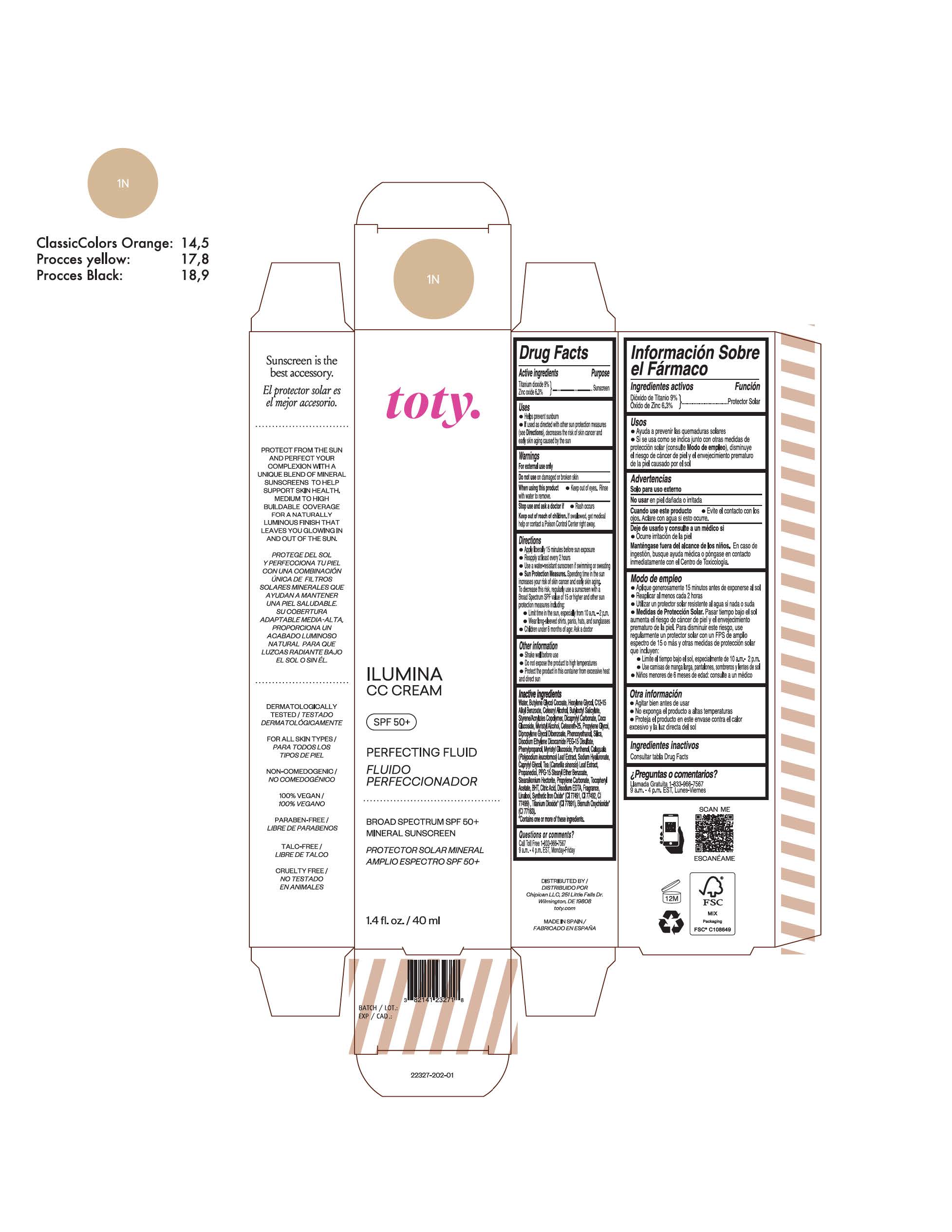
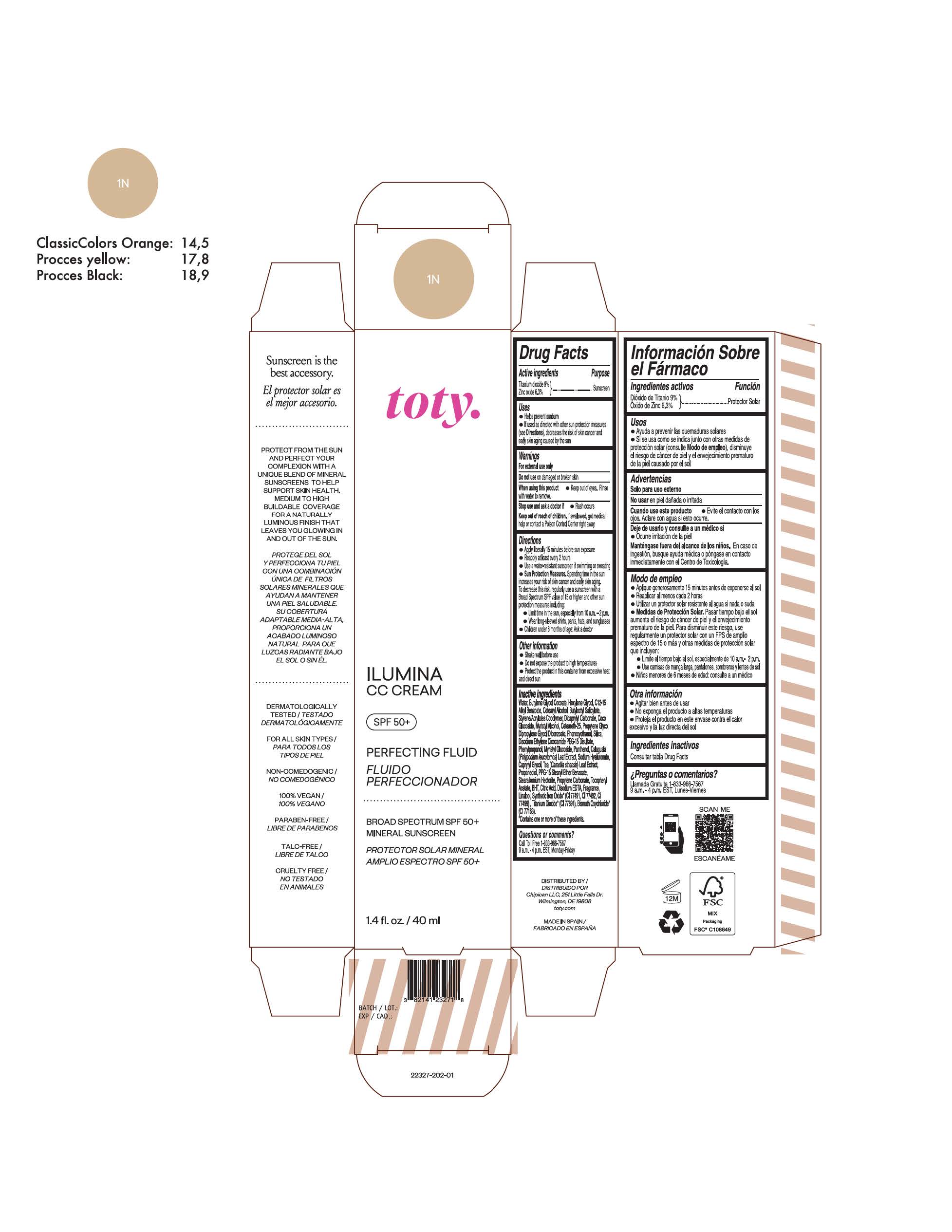
Label: TOTY ILUMINA CC CREAM 1N- titanium dioxide, zinc oxide cream
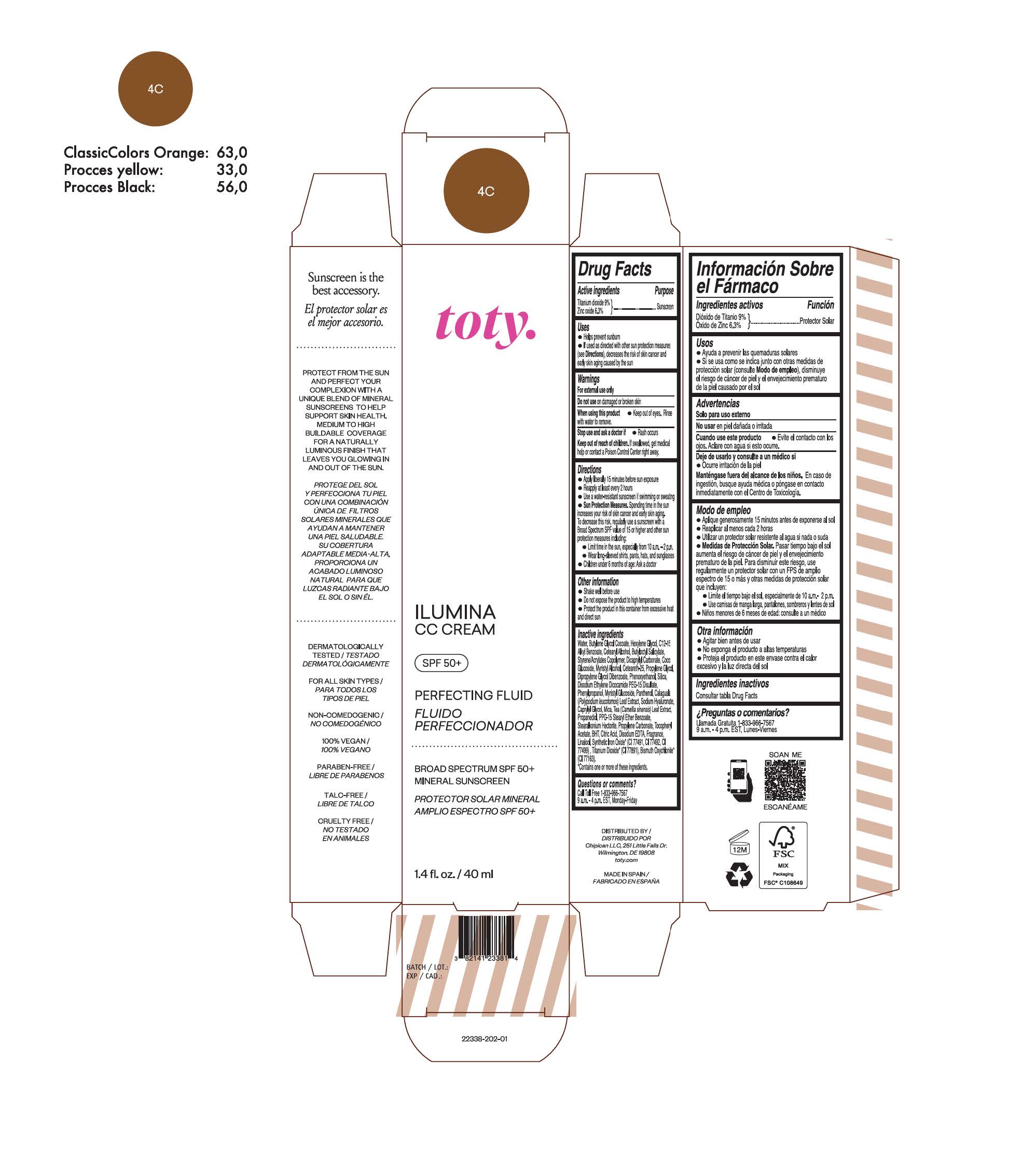
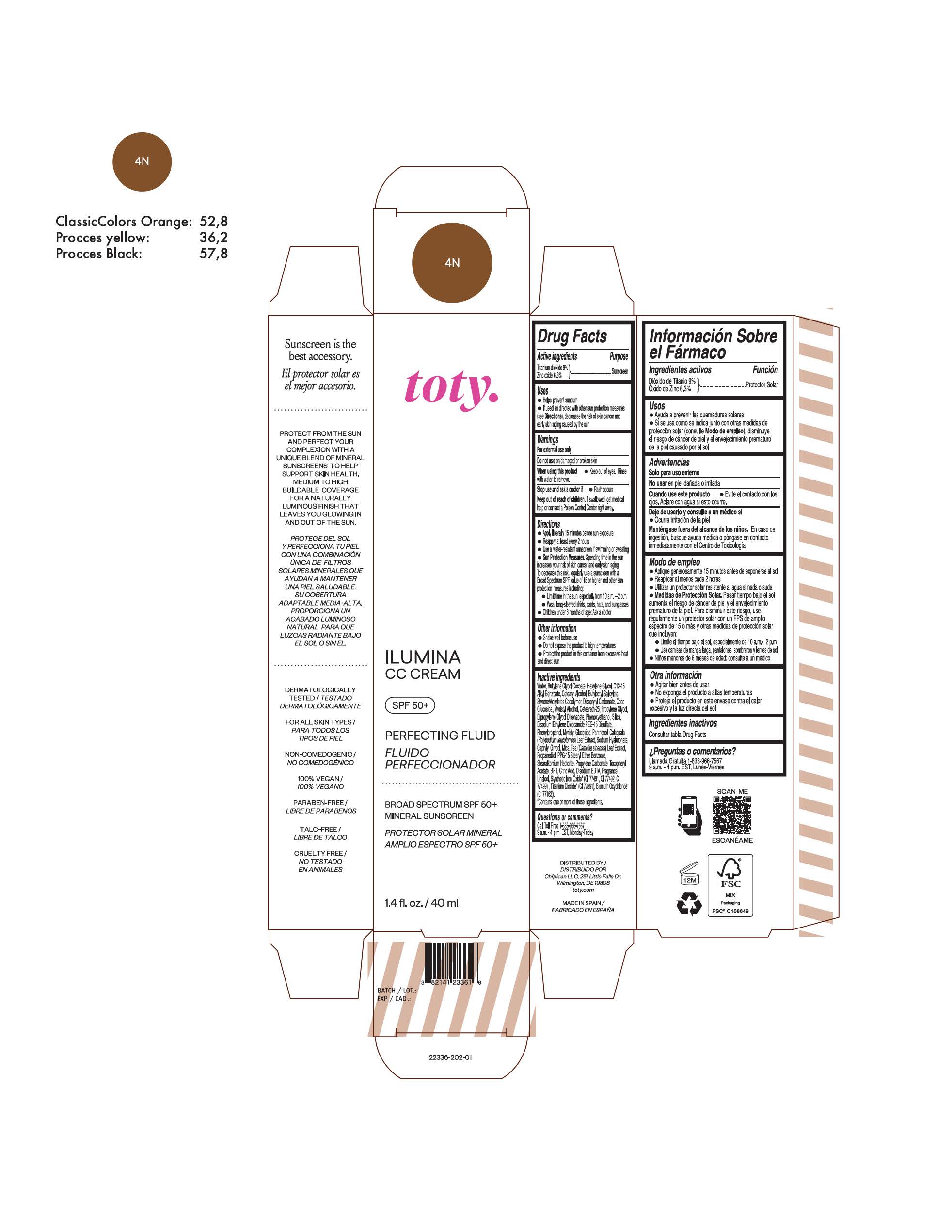
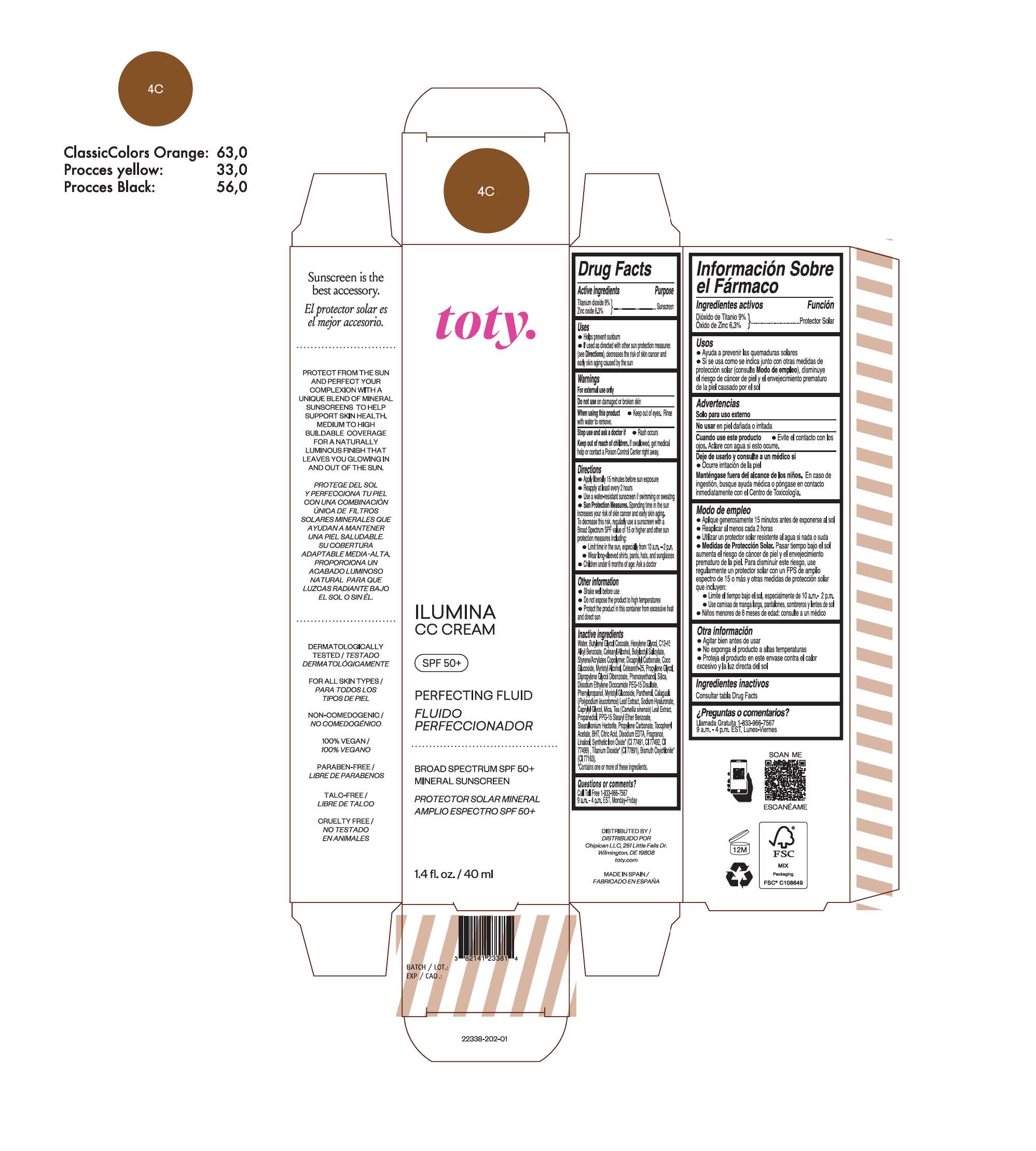
TOTY ILUMINA CC CREAM 4C- titanium dioxide, zinc oxide cream
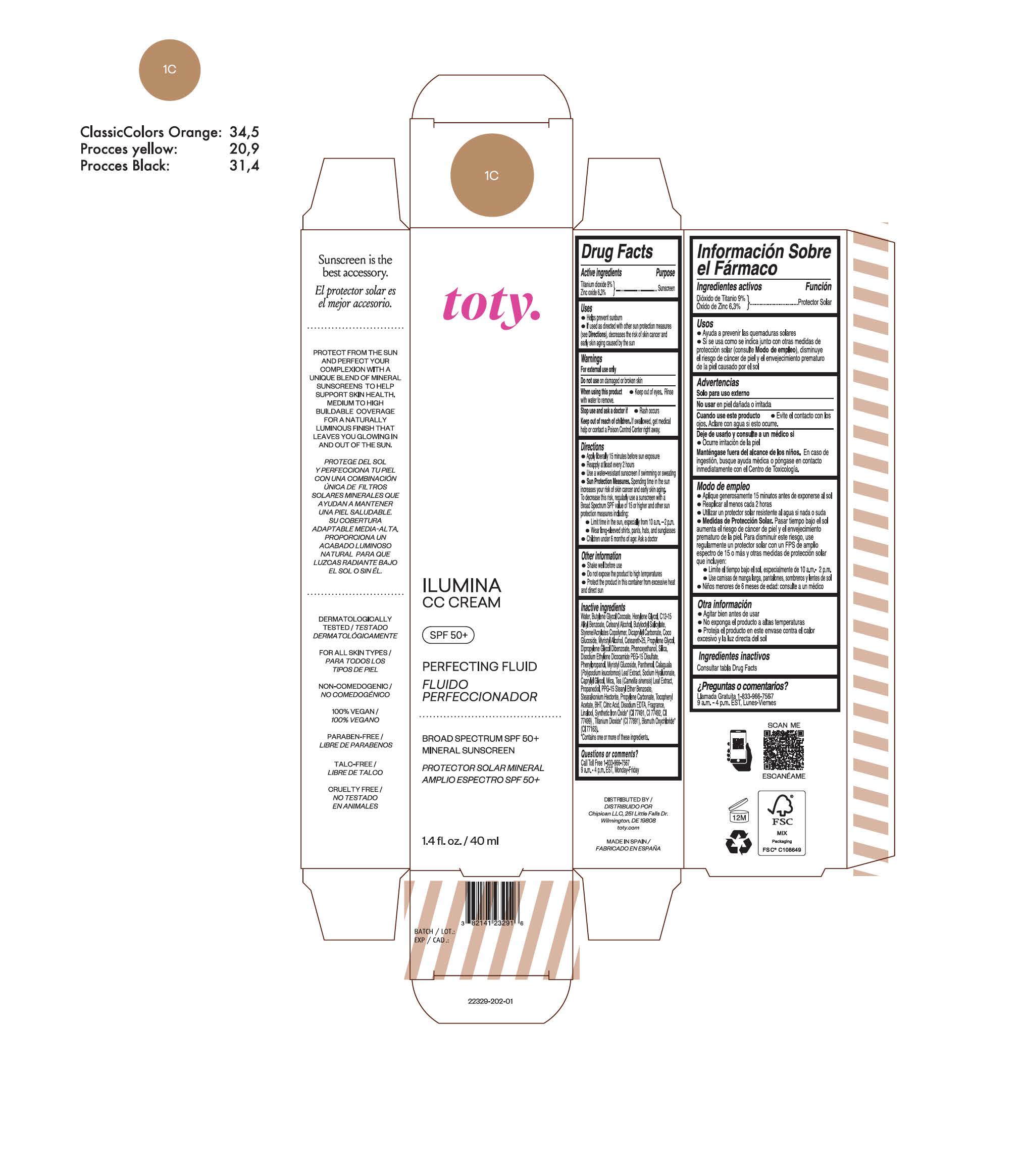
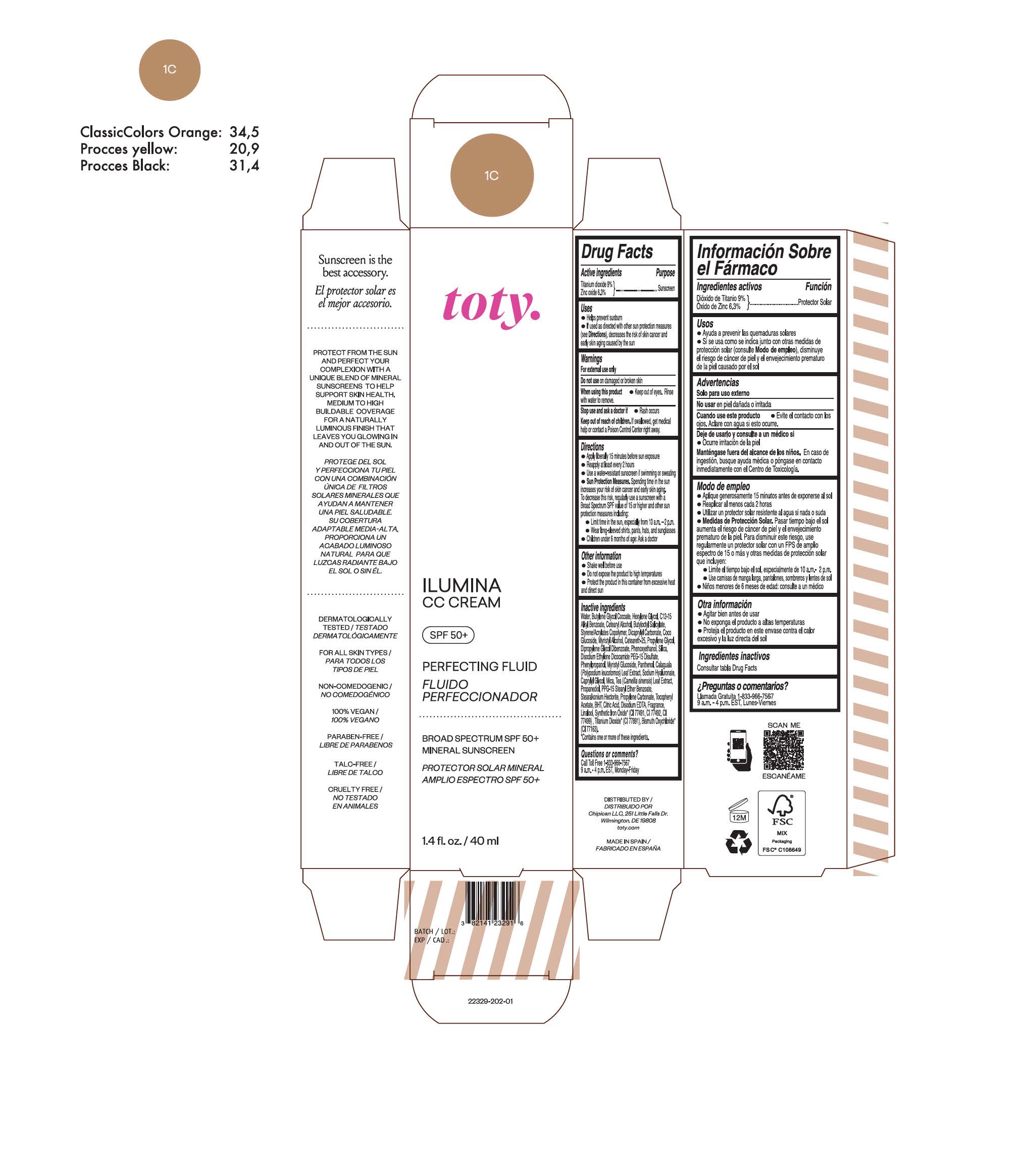
TOTY ILUMINA CC CREAM 1C- titanium dioxide, zinc oxide cream
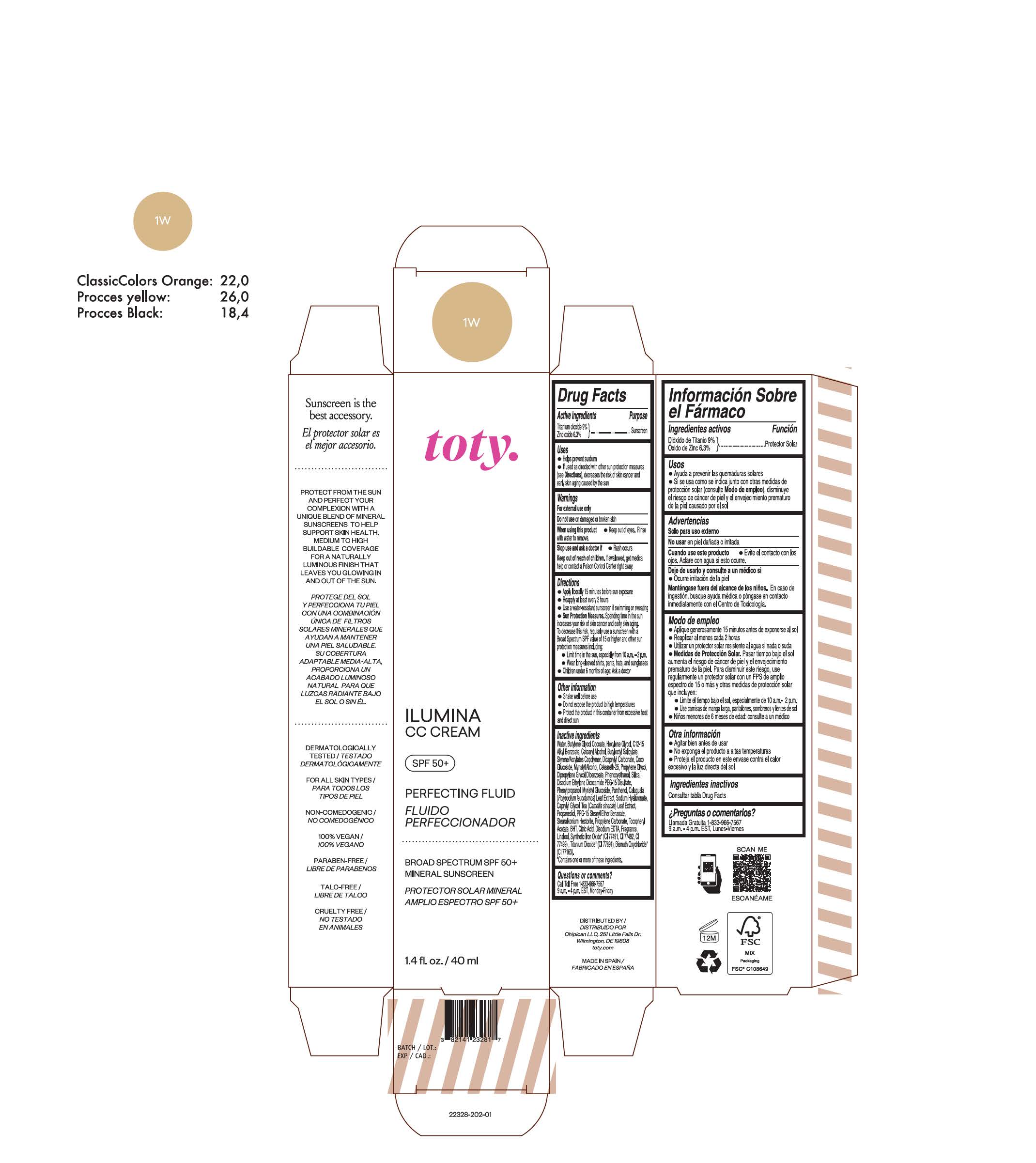
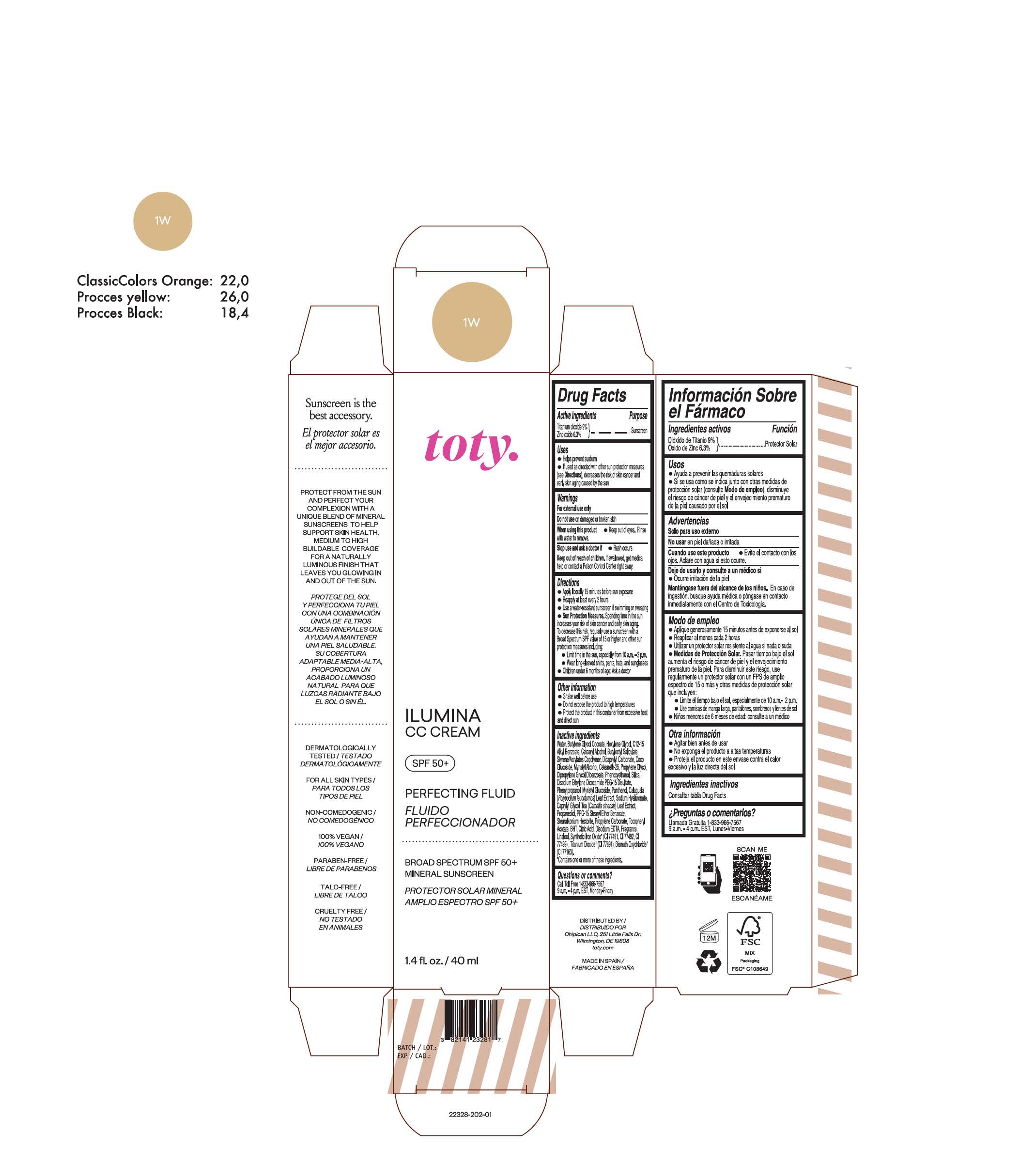
TOTY ILUMINA CC CREAM 1W- titanium dioxide, zinc oxide cream
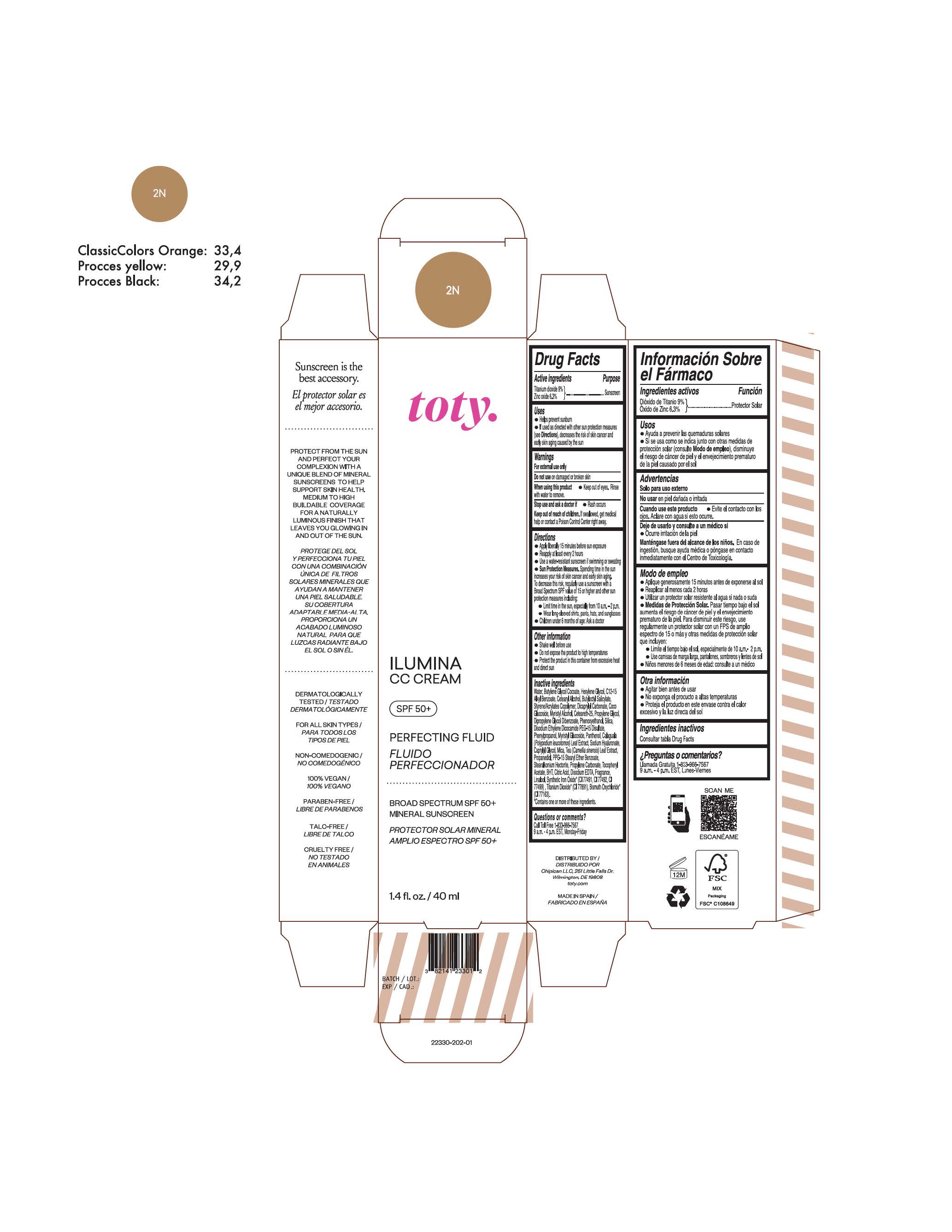
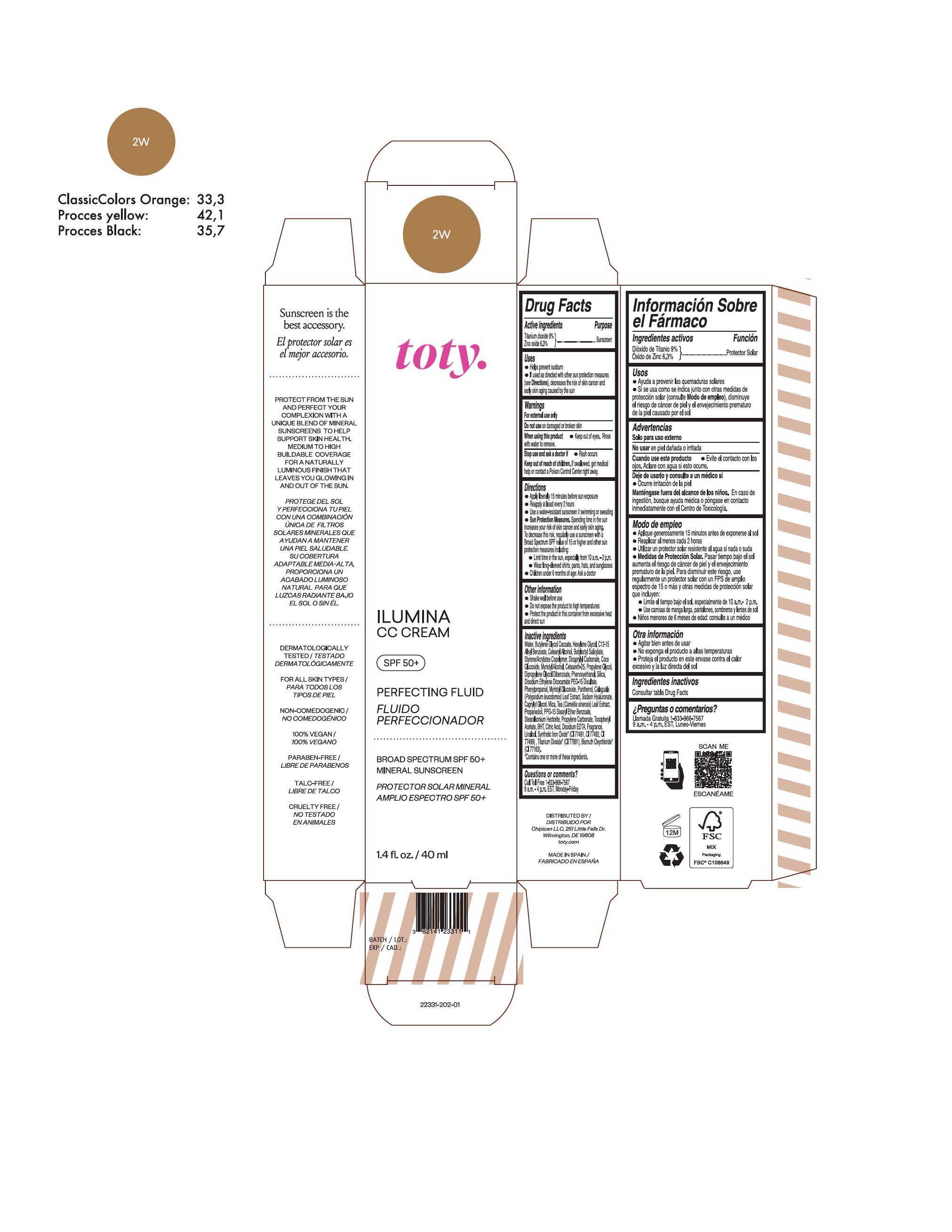
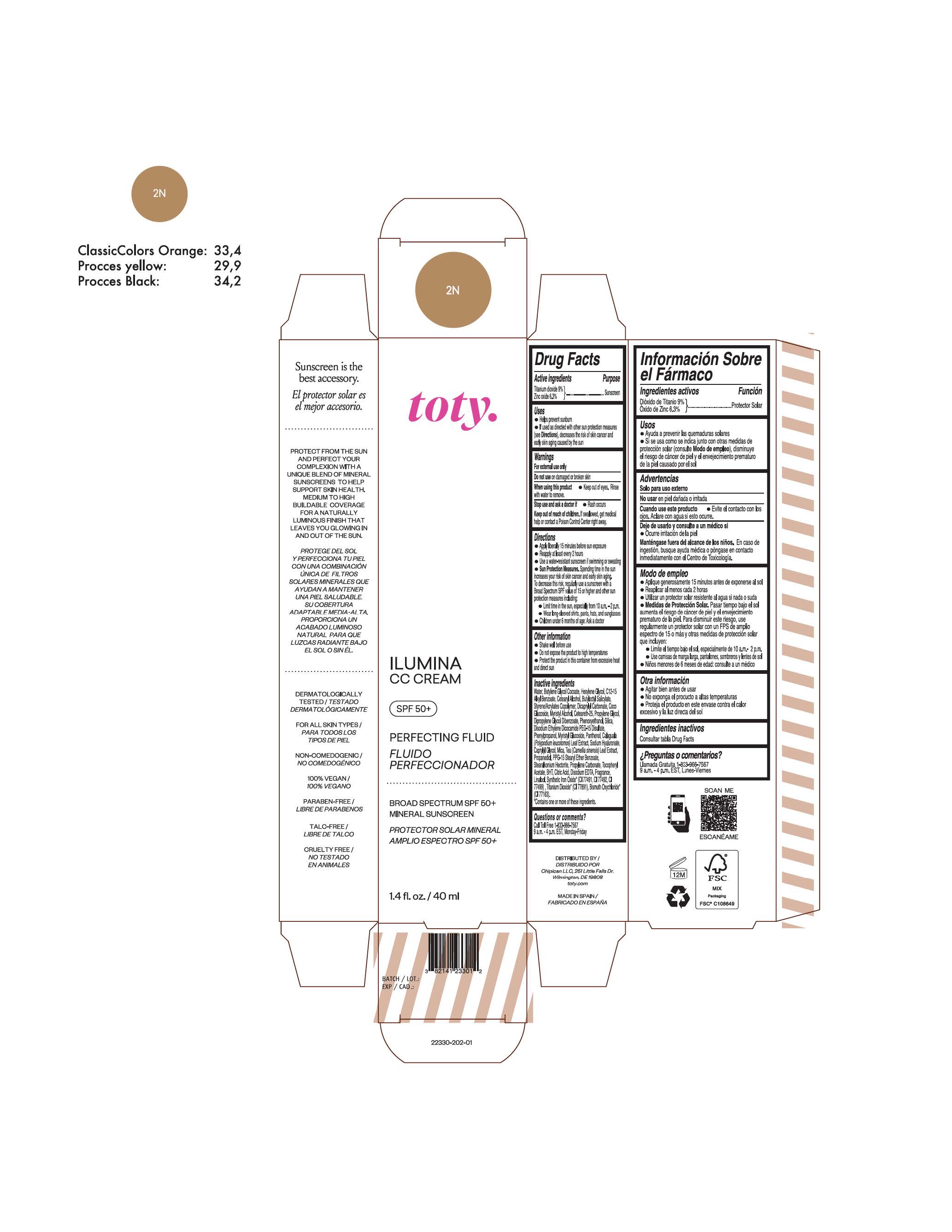
TOTY ILUMINA CC CREAM 2N- titanium dioxide, zinc oxide cream
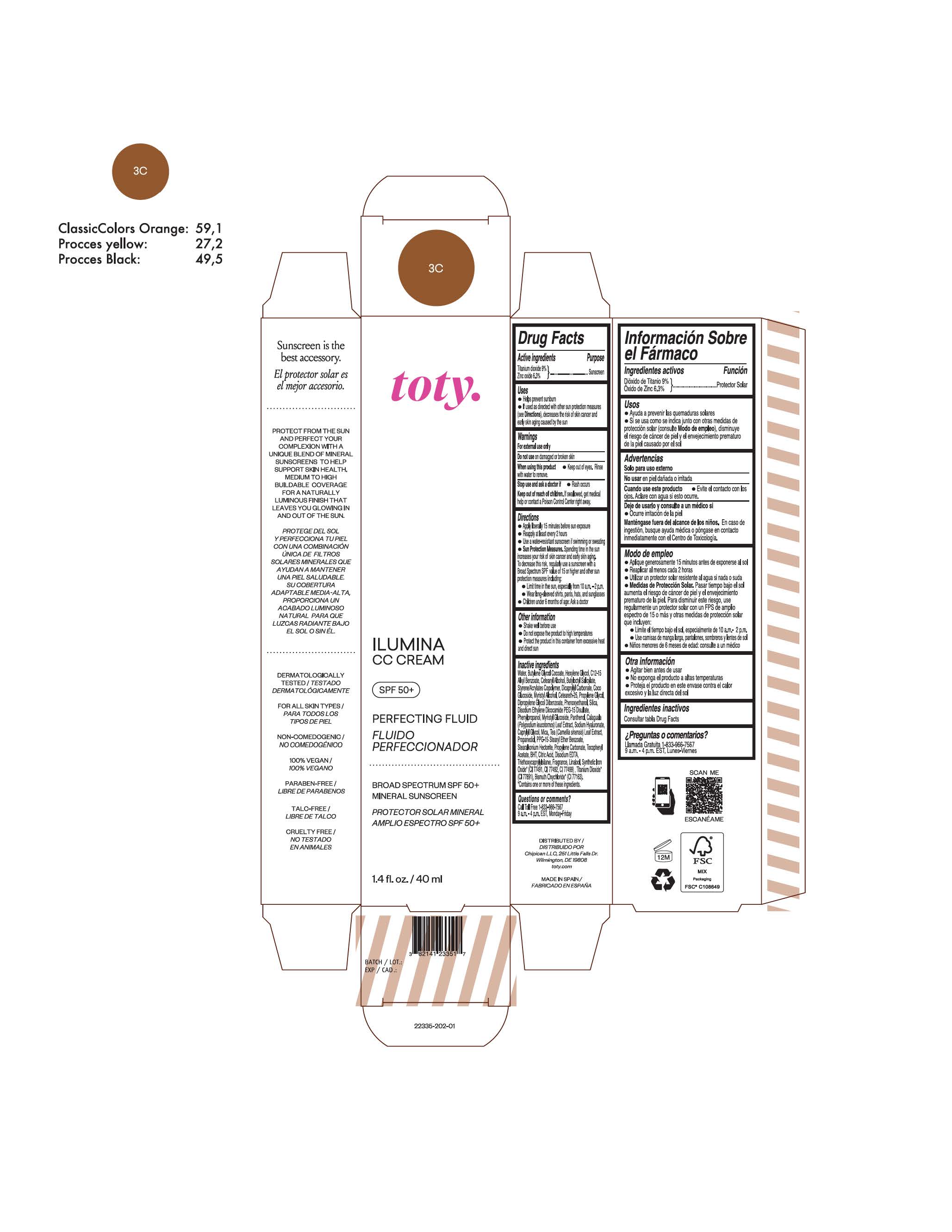
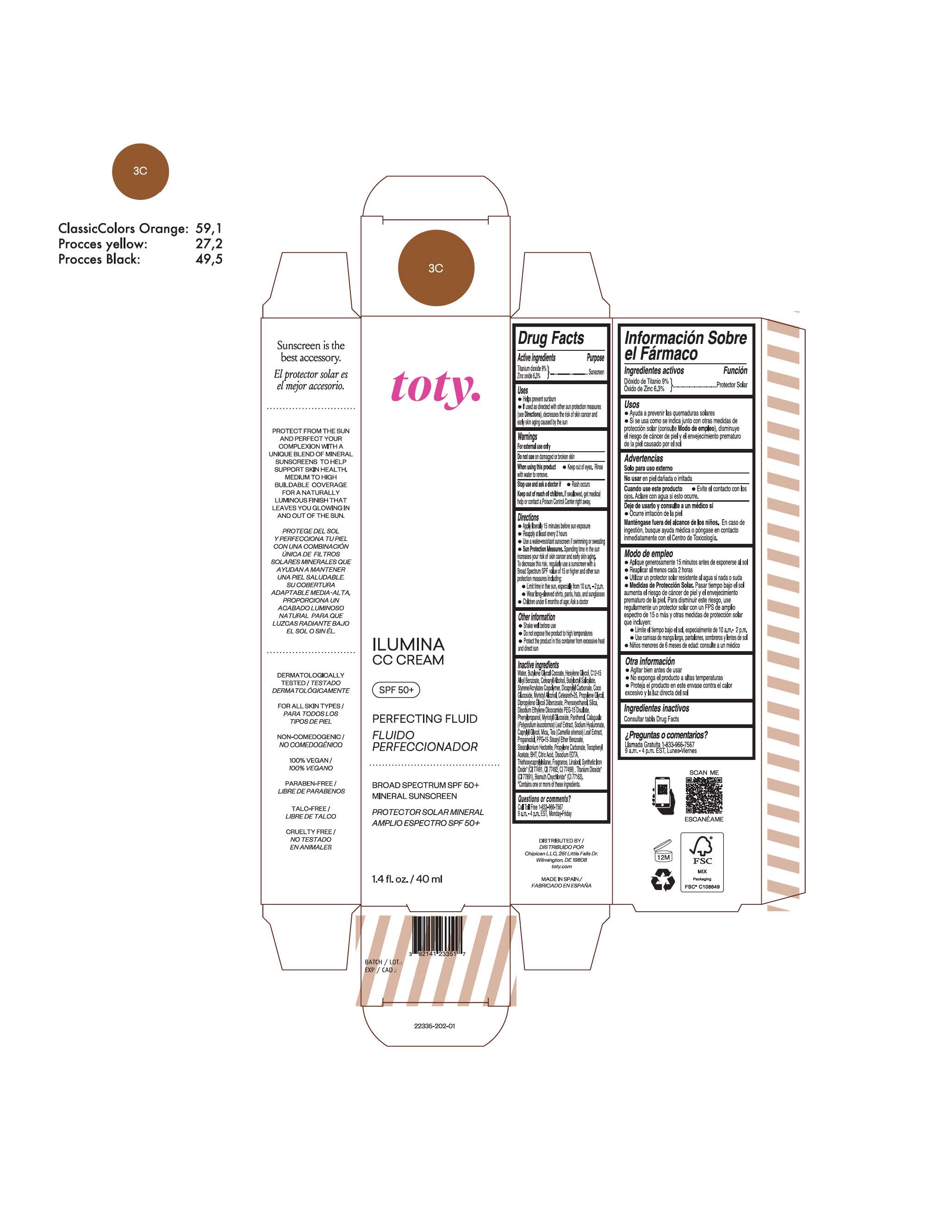
TOTY ILUMINA CC CREAM 3C- titanium dioxide, zinc oxide cream
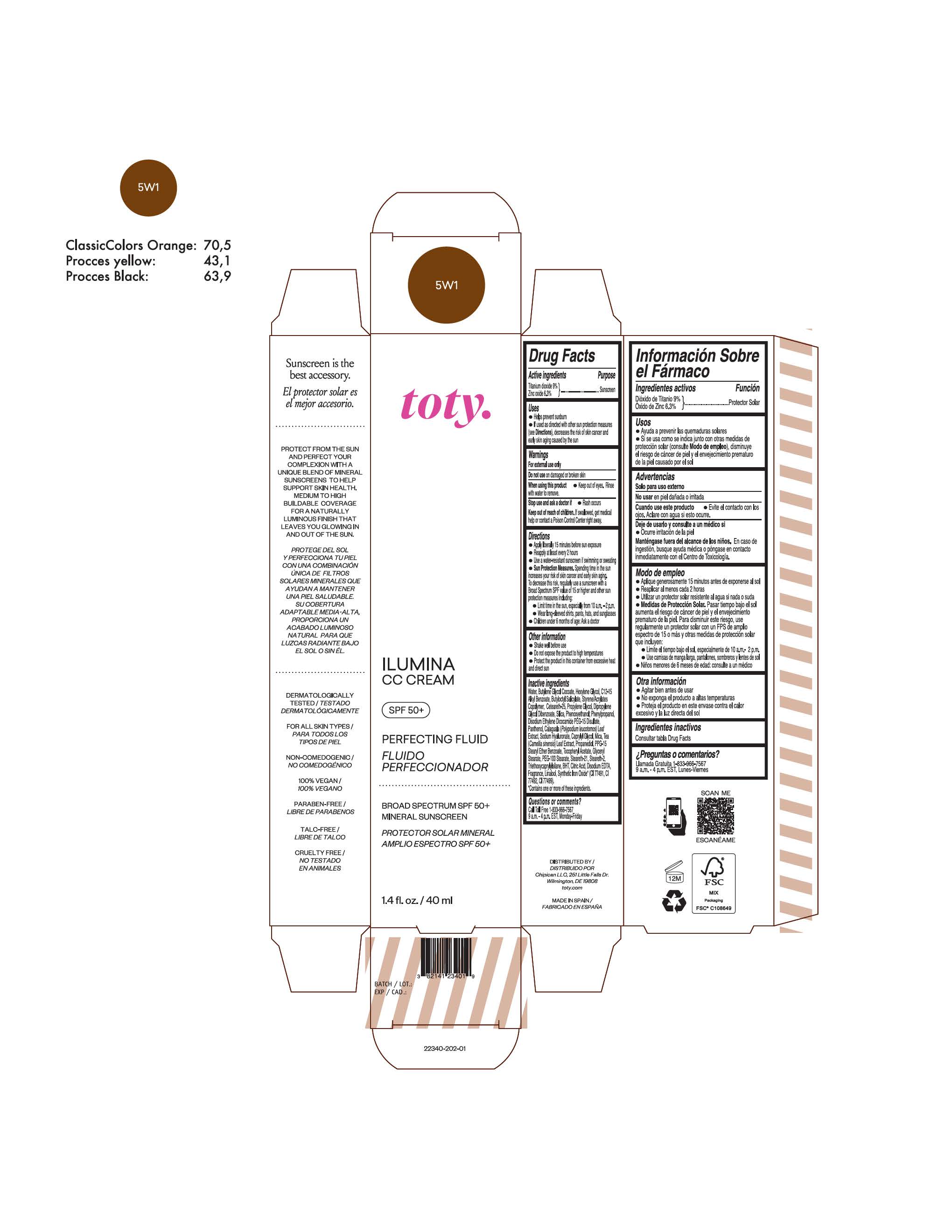
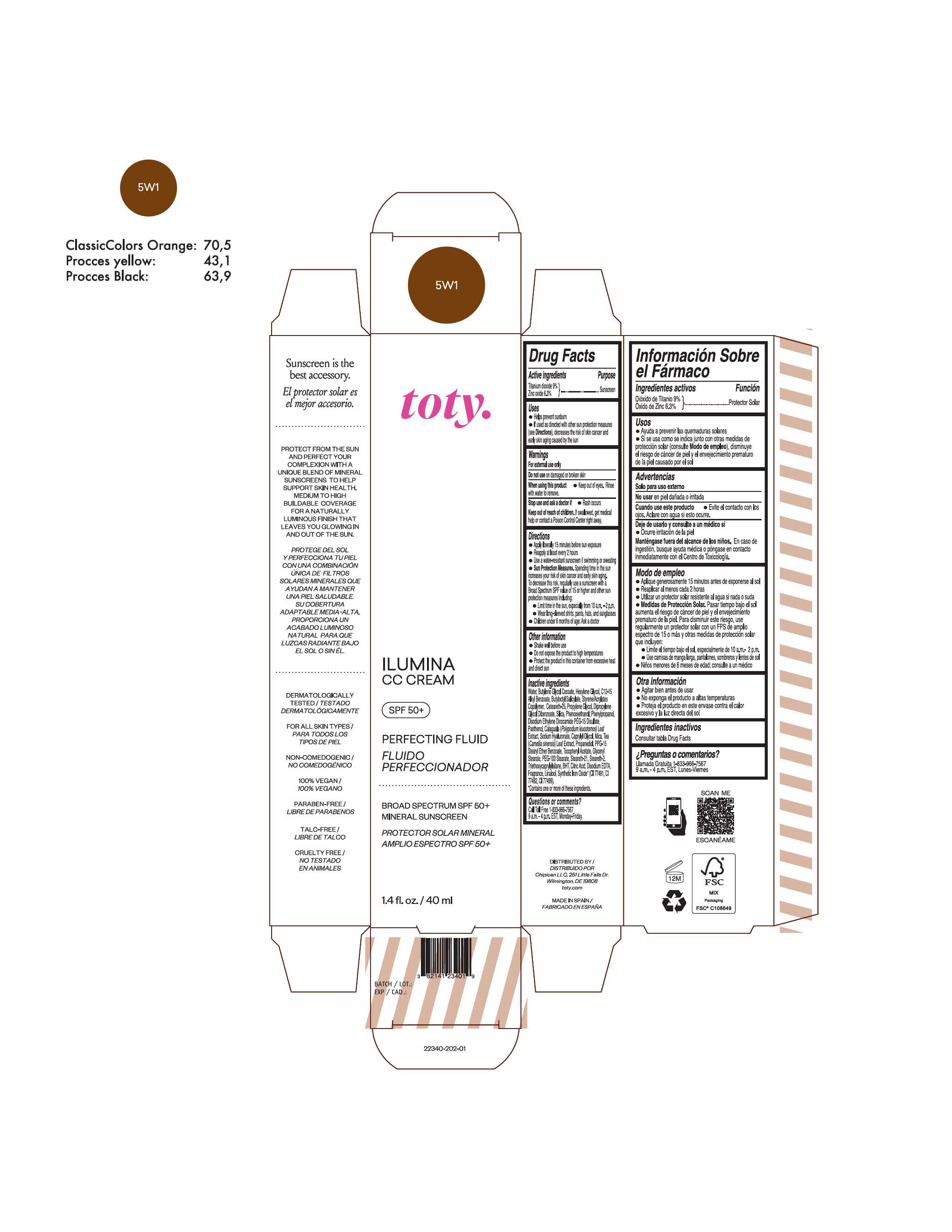
TOTY ILUMINA CC CREAM 5W1- titanium dioxide, zinc oxide cream
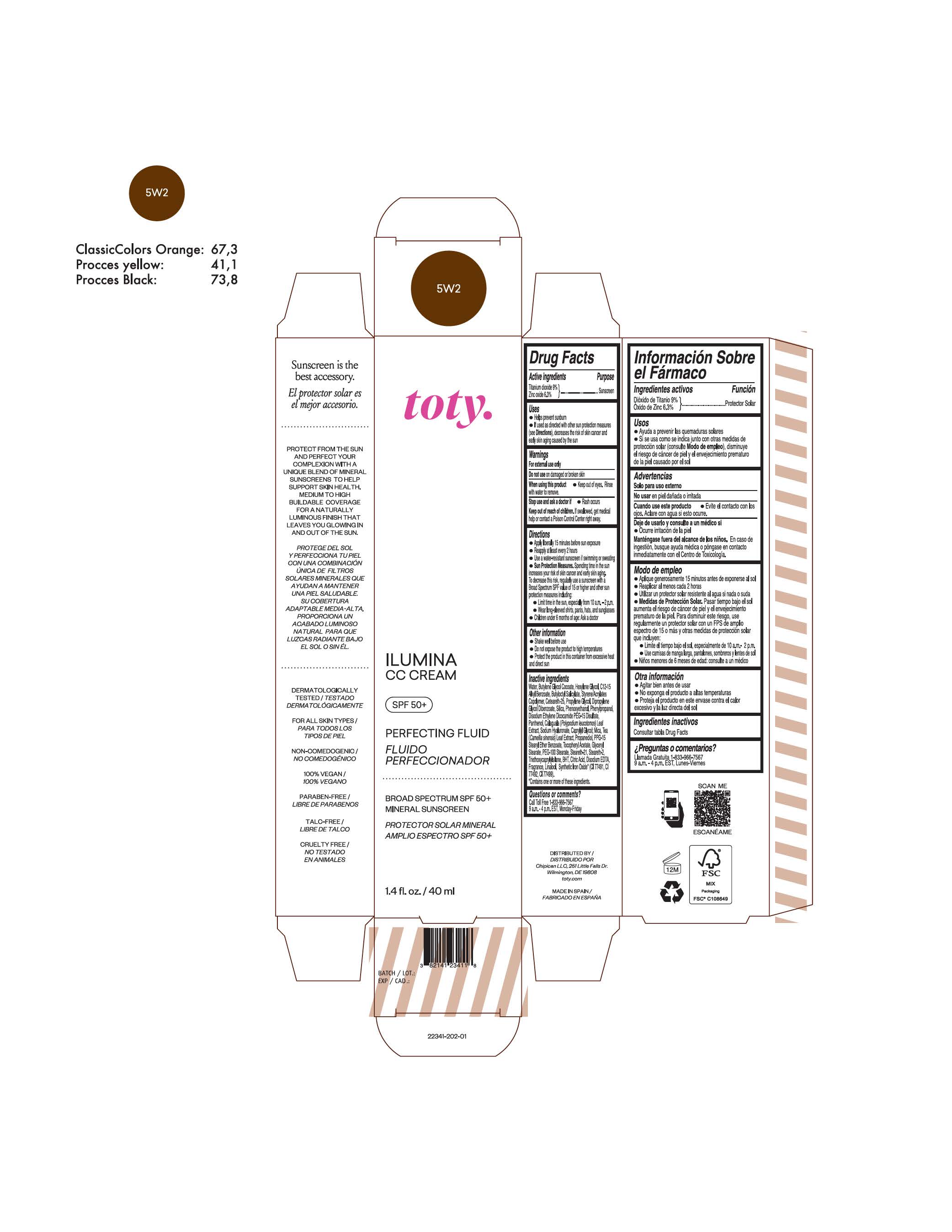
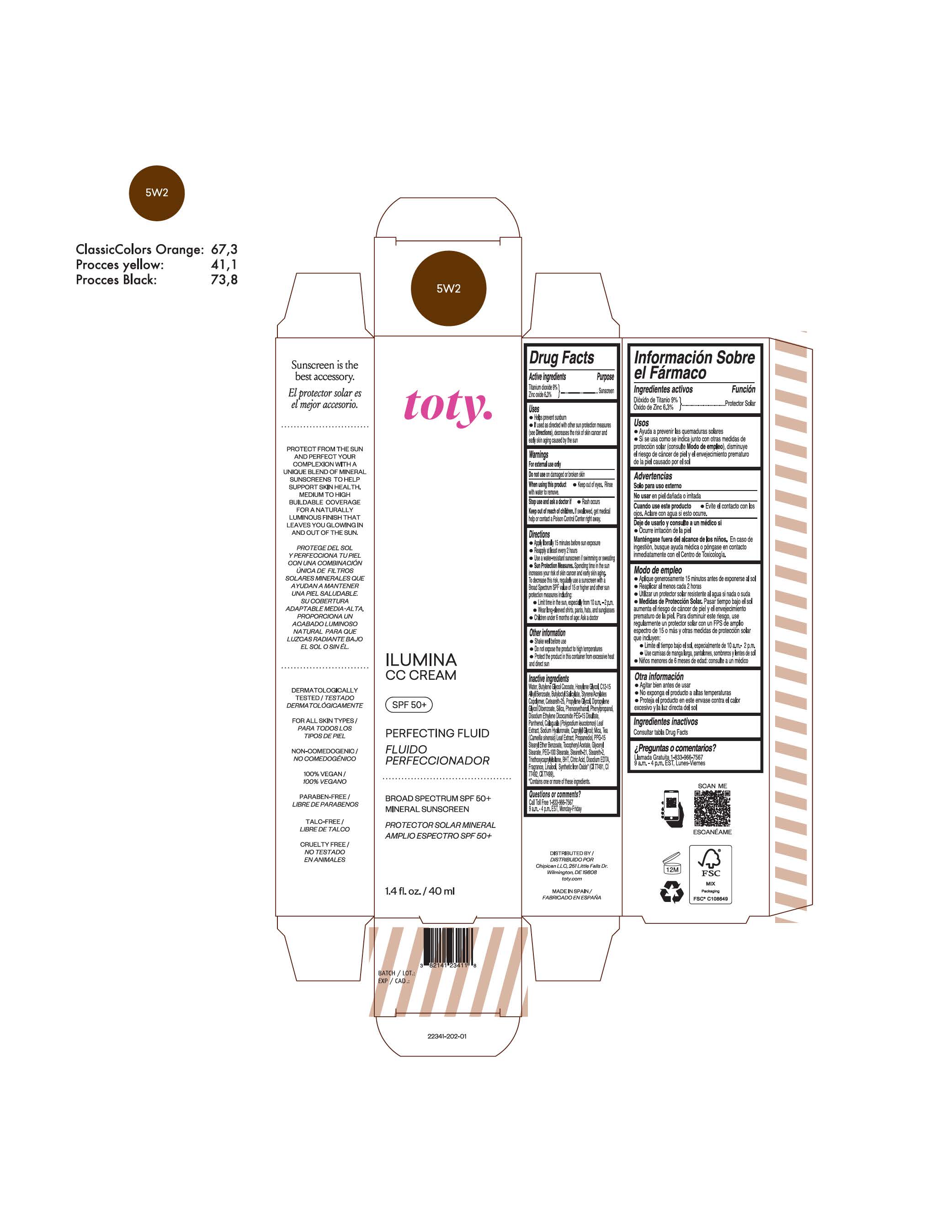
TOTY ILUMINA CC CREAM 5W2- titanium dioxide, zinc oxide cream
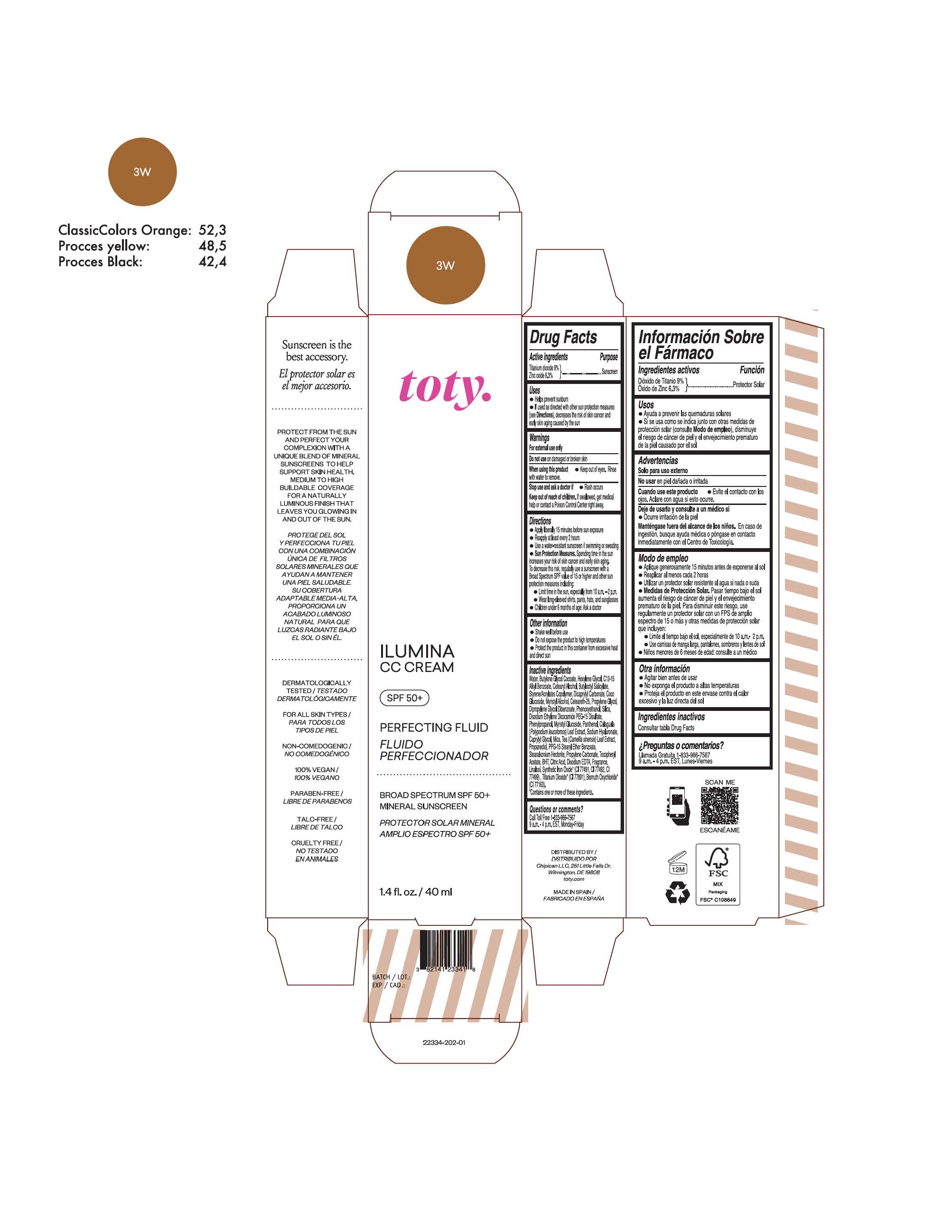
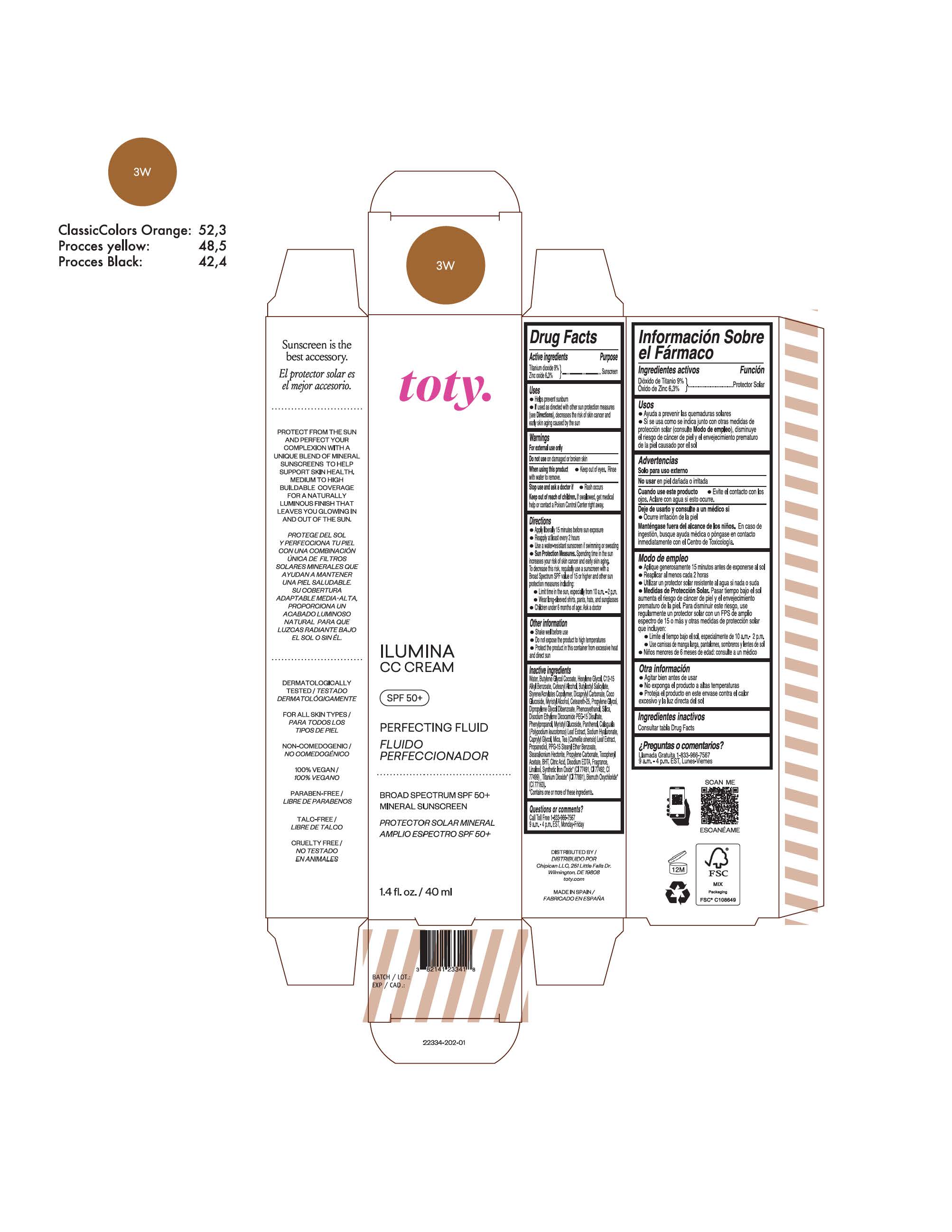
TOTY ILUMINA CC CREAM 3W- titanium dioxide, zinc oxide cream
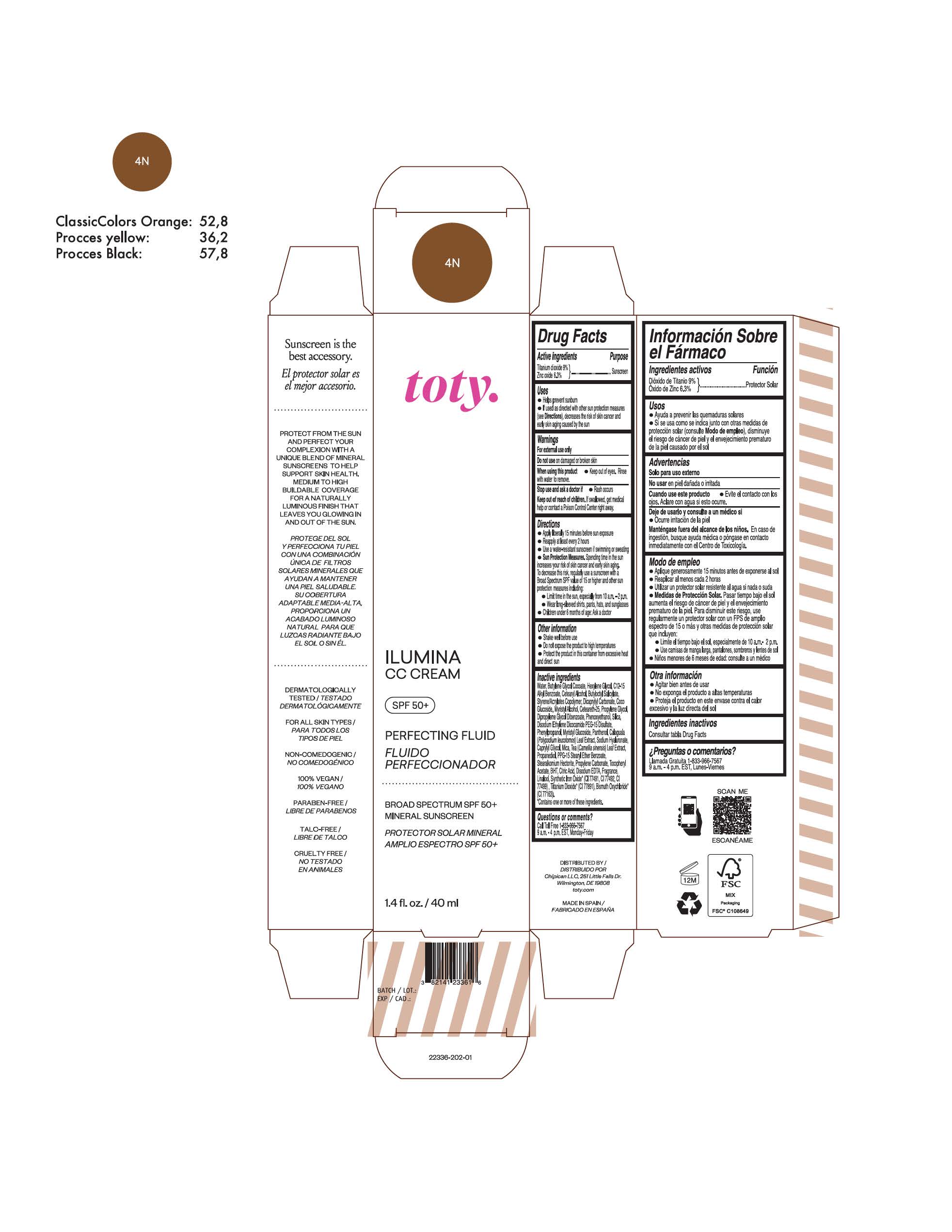
TOTY ILUMINA CC CREAM 4N- titanium dioxide, zinc oxide cream
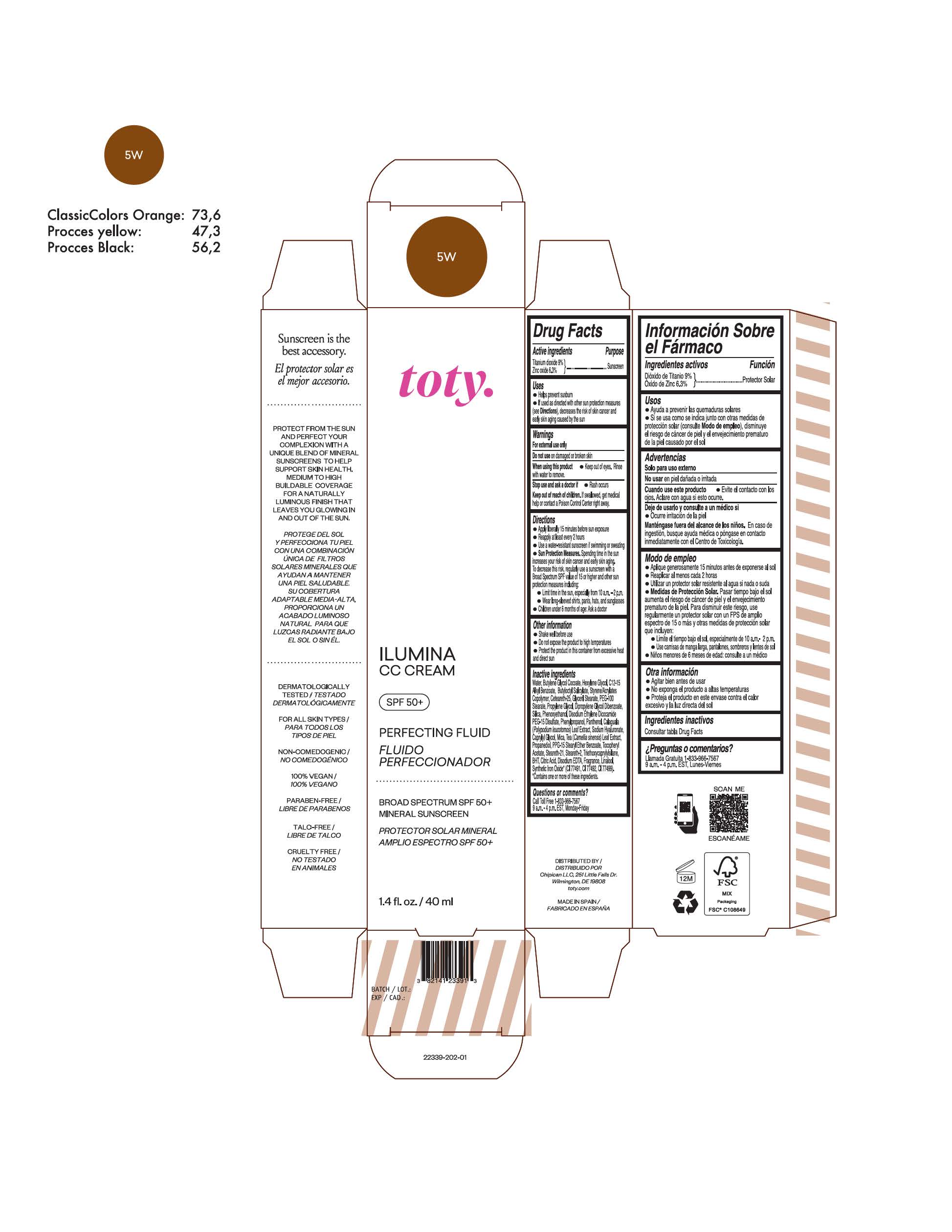
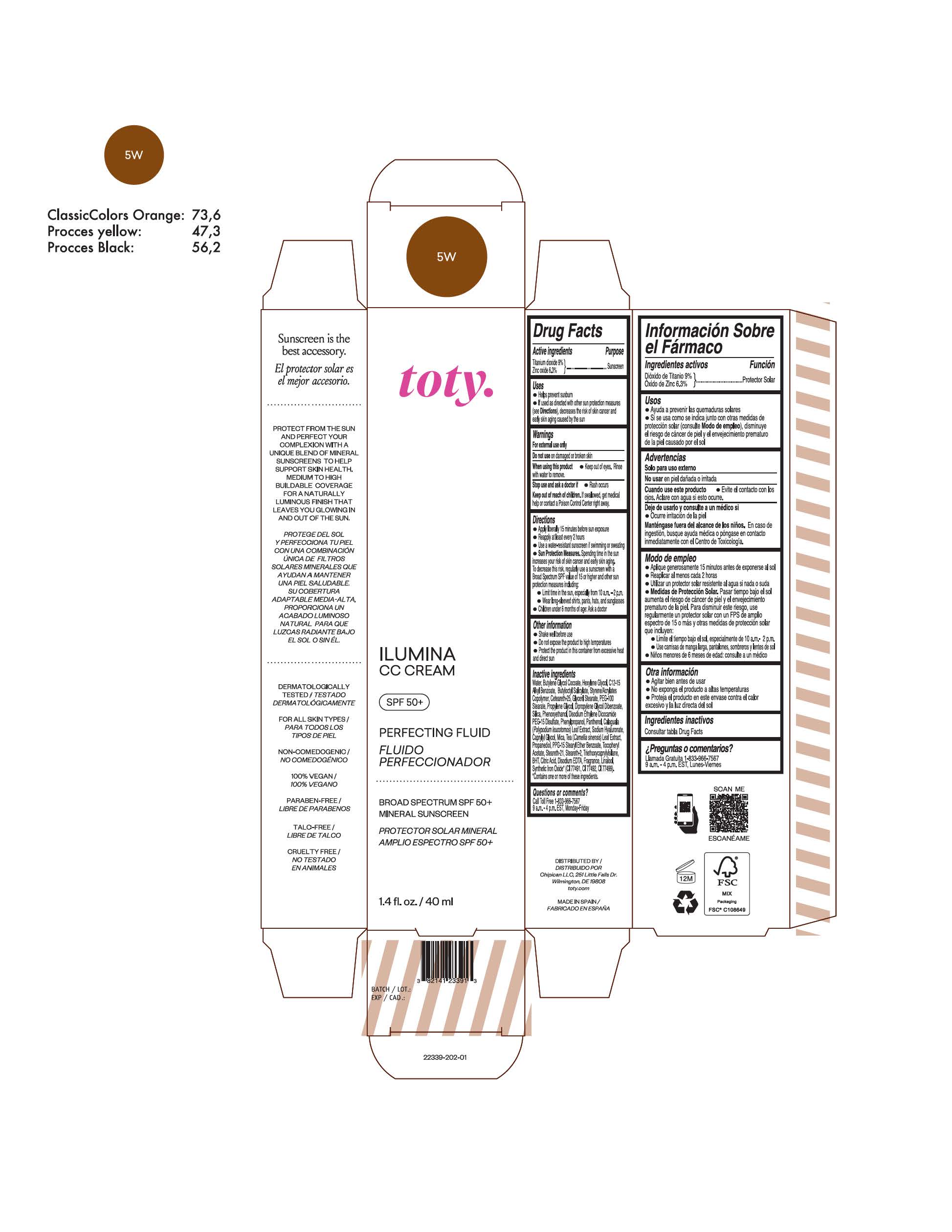
TOTY ILUMINA CC CREAM 5W- titanium dioxide, zinc oxide cream
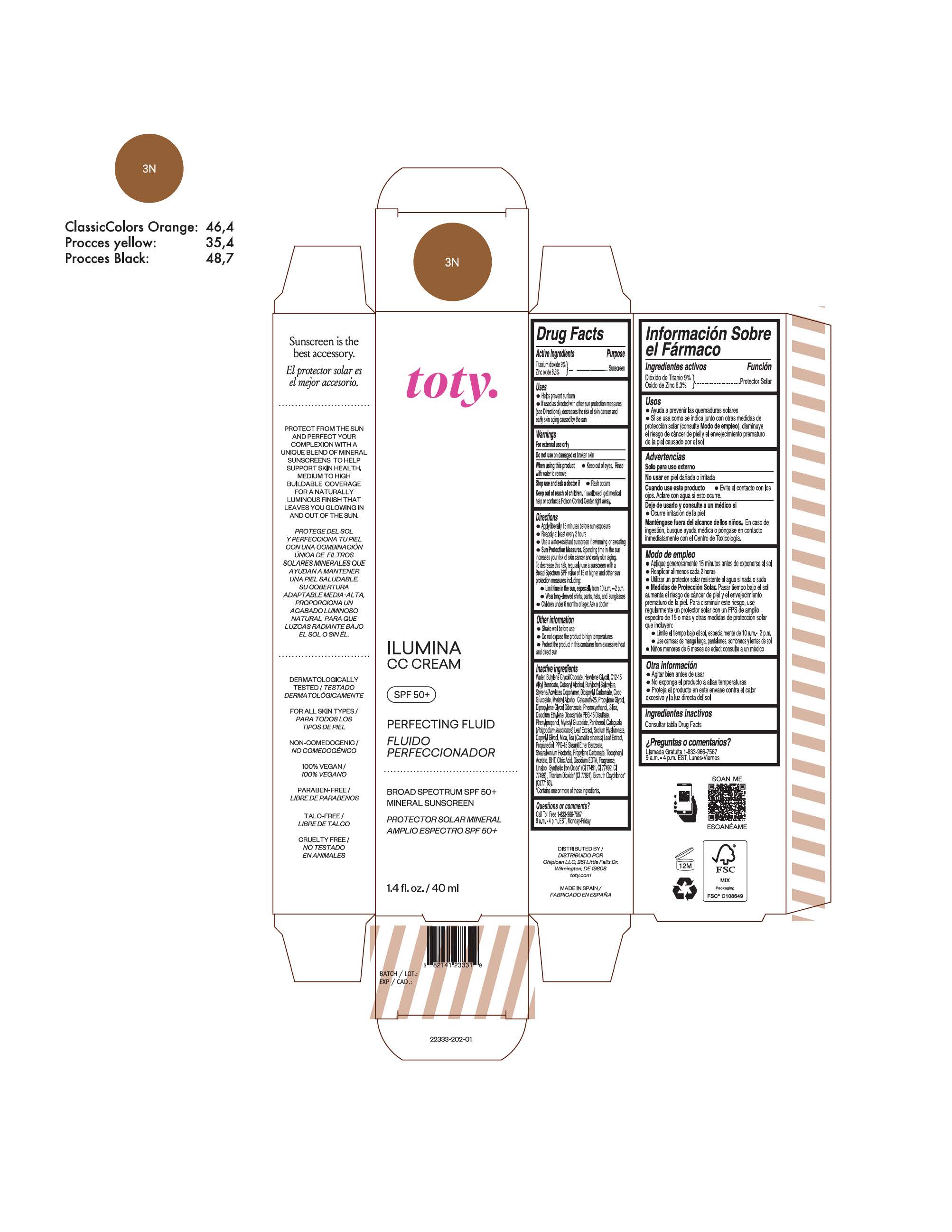
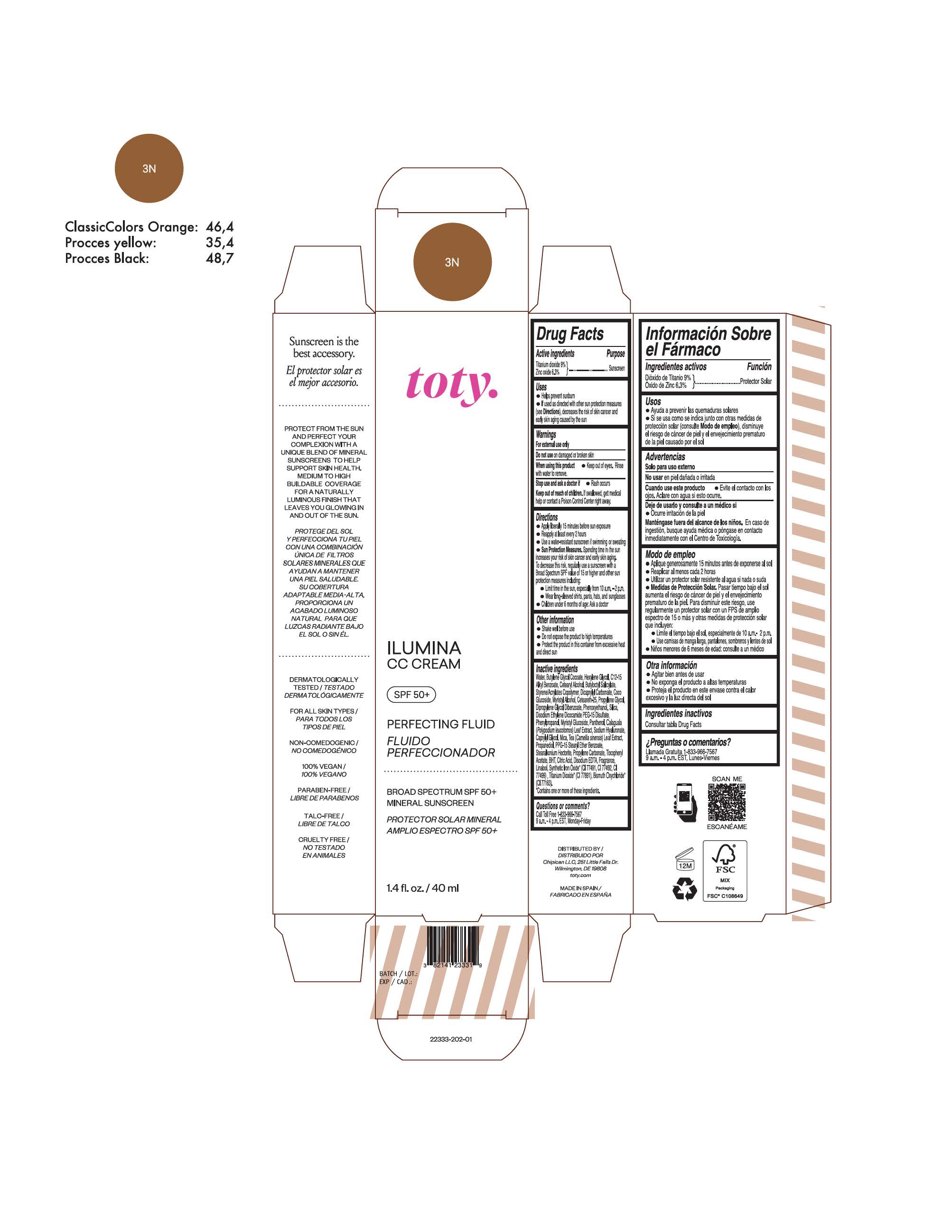
TOTY ILUMINA CC CREAM 3N- titanium dioxide, zinc oxide cream
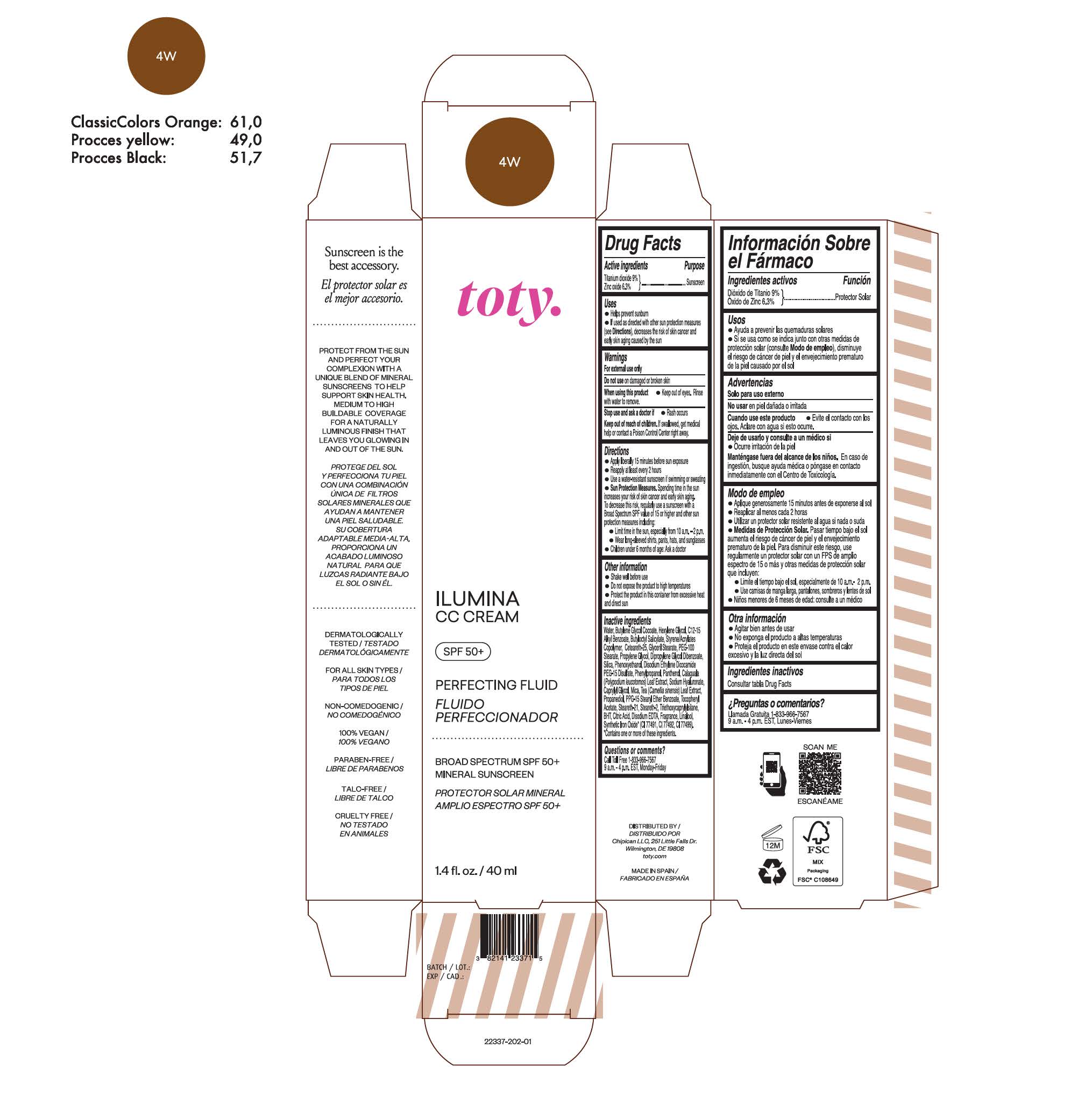
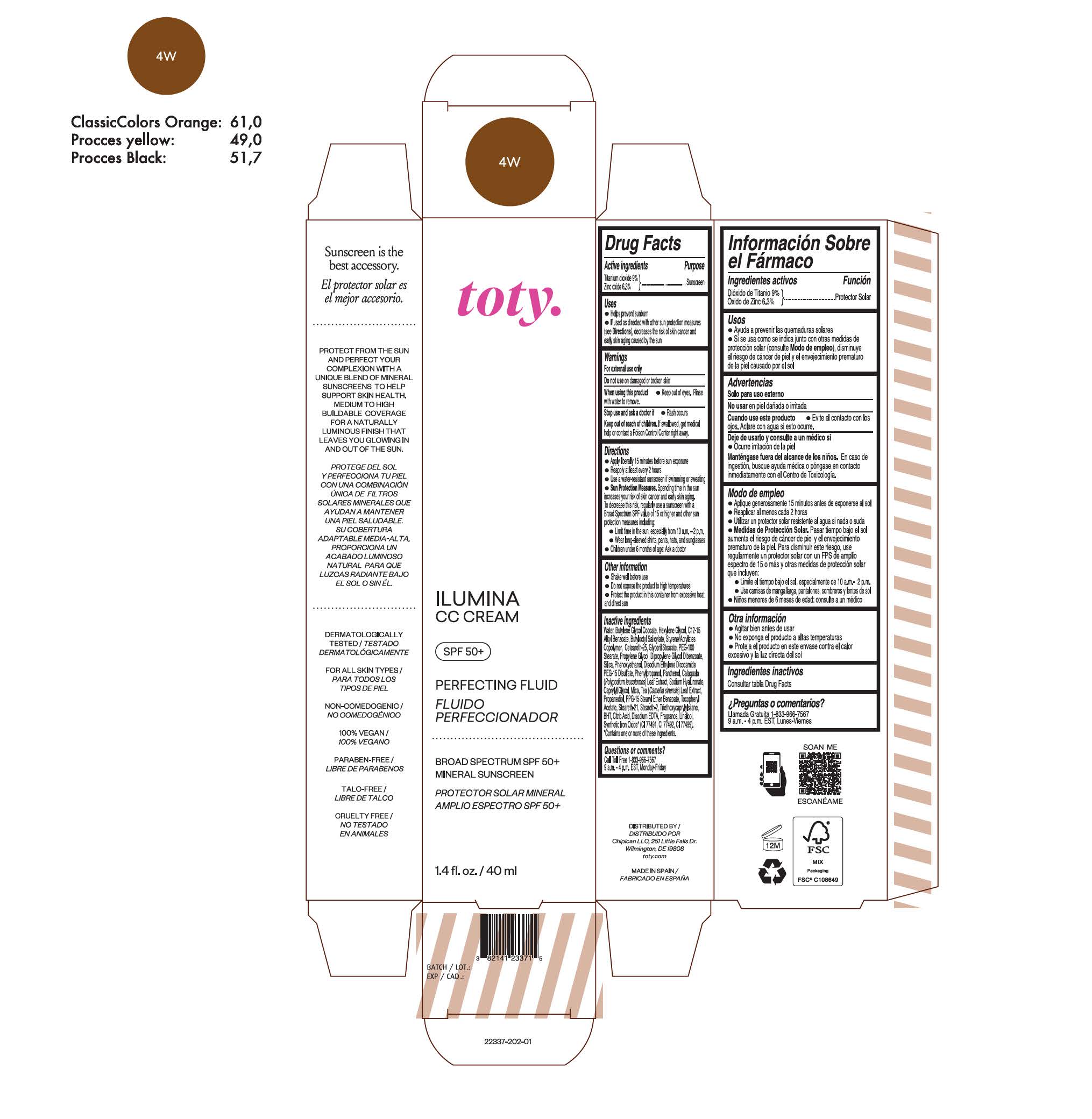
TOTY ILUMINA CC CREAM 4W- titanium dioxide, zinc oxide cream
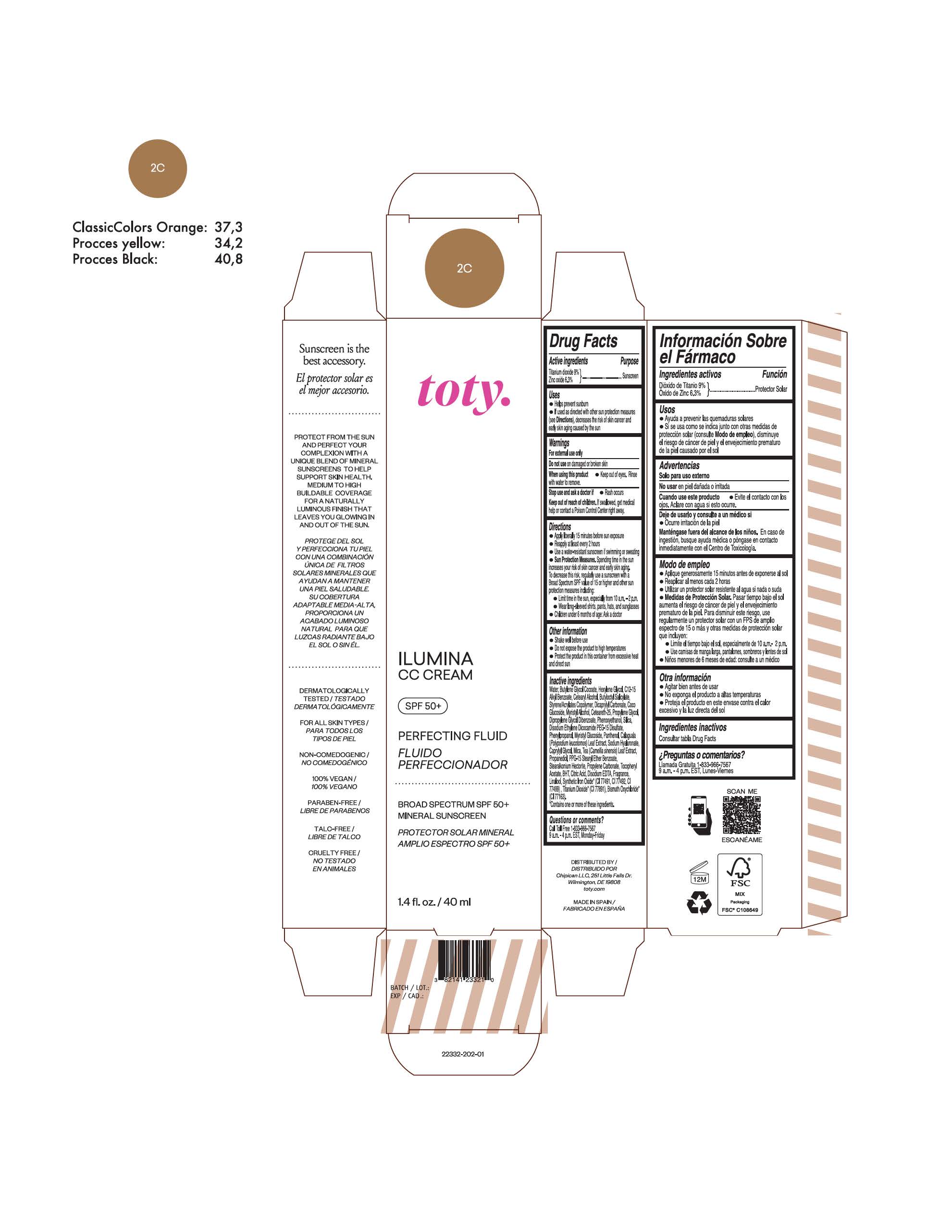
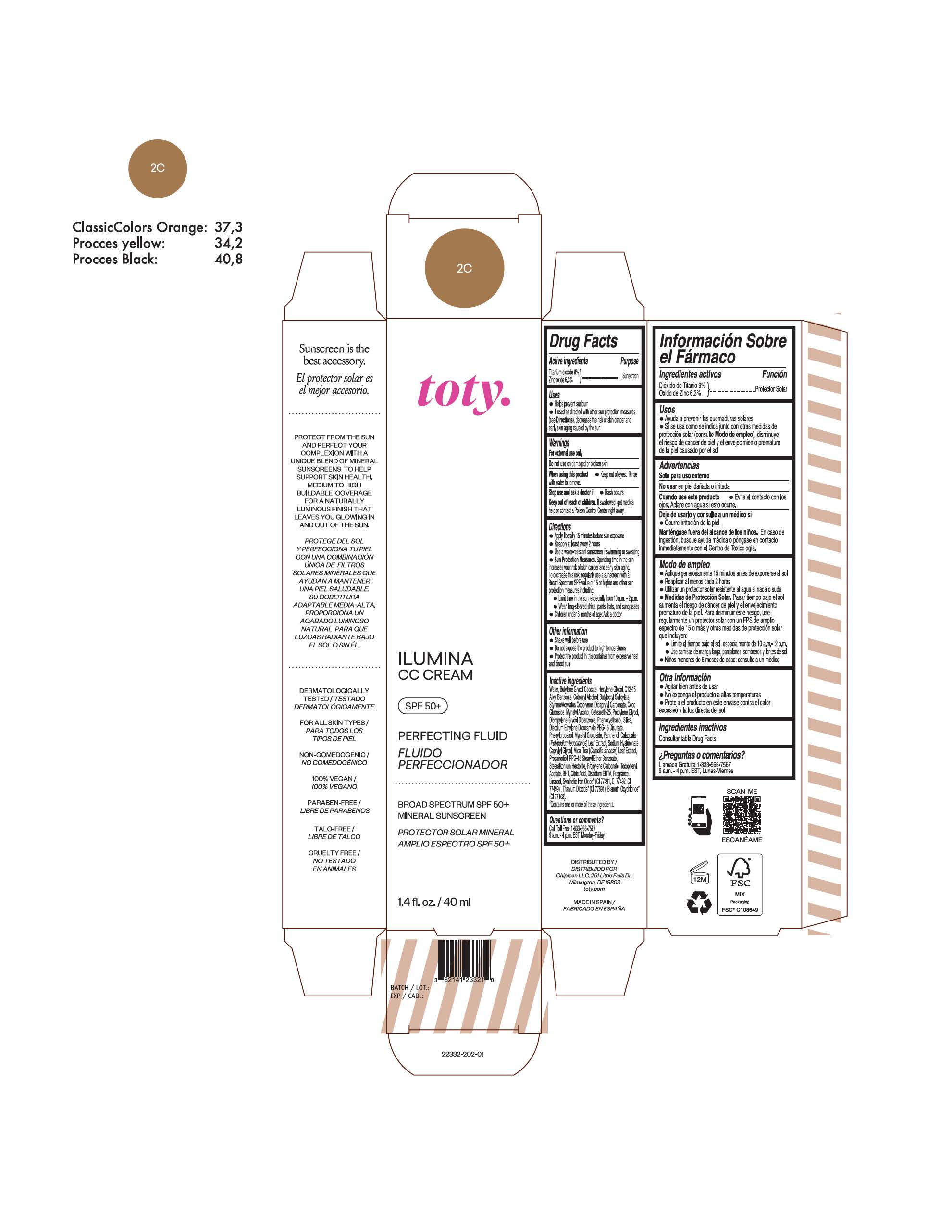
TOTY ILUMINA CC CREAM 2C- titanium dioxide, zinc oxide cream
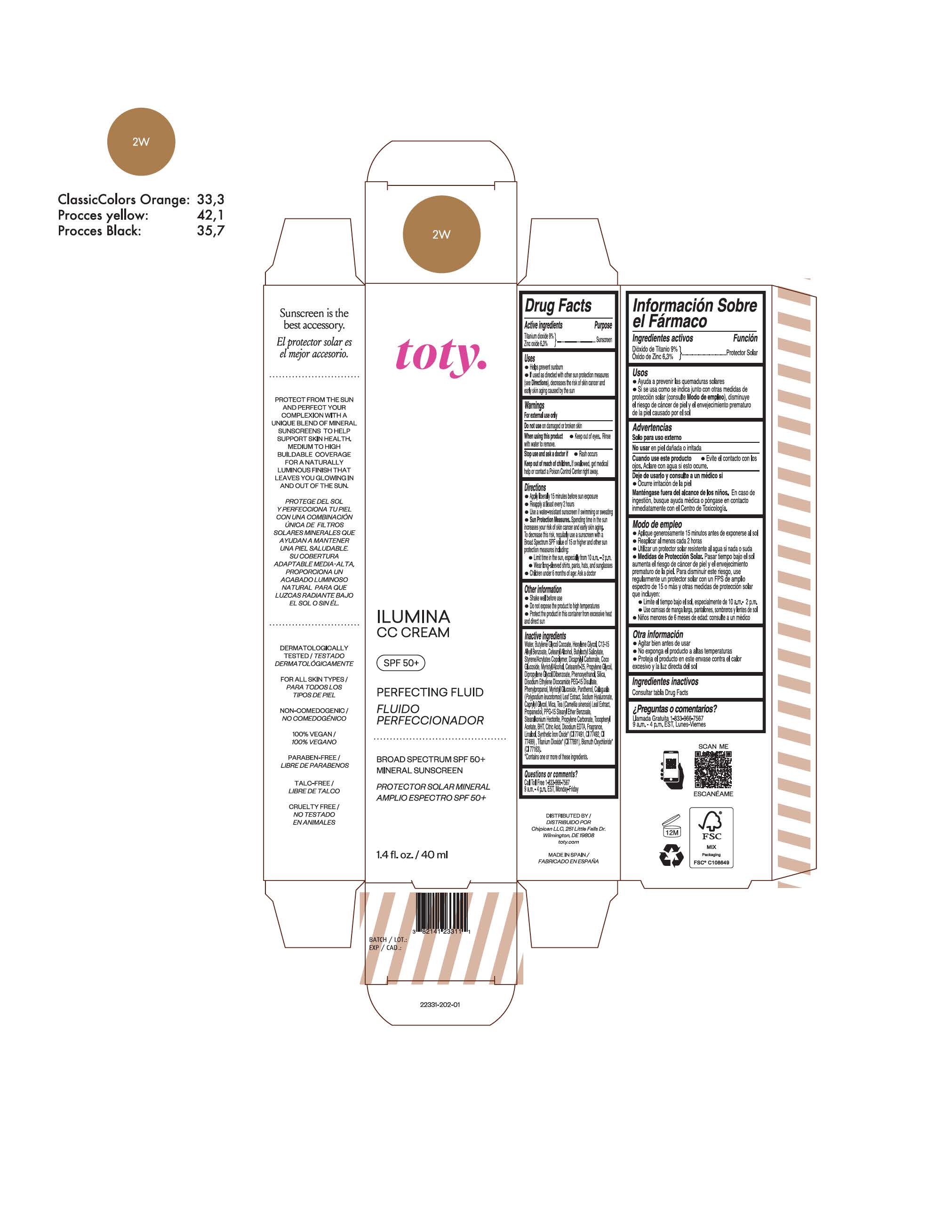
TOTY ILUMINA CC CREAM 2W- titanium dioxide, zinc oxide cream
-
NDC Code(s):
82141-2327-1,
82141-2328-1,
82141-2329-1,
82141-2330-1, view more82141-2331-1, 82141-2332-1, 82141-2333-1, 82141-2334-1, 82141-2335-1, 82141-2336-1, 82141-2337-1, 82141-2338-1, 82141-2339-1, 82141-2340-1, 82141-2341-1
- Packager: Chipican LLC
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated September 27, 2022
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
- Uses
- Warnings
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
- Apply liberally 15 minutes behore sun exposure
- Reapply at least every 2 hours
- Use a water-resistant sunscreen if swimmimg or sweating
- Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- Limit time in the sun, especially from 10 a.m. - 2 p.m
- Wear long-sleeved shirts, pants, hats, and sunglasses
- Children under 6 months of age: Ask a doctor.
- Other Information
-
Inactive Ingredients
NDCs 82141-2327-1, 82141-2328-1
Water, Butylene Glycol Cocoate,Hexylene Glycol, C12-15
Alkyl Benzoate, Cetearyl Alcohol, Butyloctyl Salicylate,
Styrene/Acrylates Copolymer, Dicaprylyl Carbonate, Coco
Glucoside, Myristyl Alcohol, Ceteareth-25, Propylene Glycol,
Dipropylene Glycol Dibenzoate, Phenoxyethanol, Silica,
Disodium Ethylene Dicocamide PEG-15 Disulfate,
Phenylpropanol, Myristyl Glucoside, Panthenol, Calaguala
( Polypodium leucotomos) Leaf Extract, Sodium Hyaluronate,
Caprylyl Glycol, Tea ( Camellia sinensis) Leaf Extract,
Propanediol, PPG-15 Stearyl Ether Benzoate,
Stearalkonium Hectorite, Propylene Carbonate, Tocopheryl
Acetate, BHT, Citric Acid, Disodium EDTA, Fragrance,
Linalool, Synthetic Iron Oxide* (CI 77491, CI 77492, CI
77499), Titanium Dioxide* (CI 77891), Bismuth Oxychloride*
(CI 77163).
* Contains one or more of these ingredients.
NDCs 82141-2329-1, 82141-2330-1, 82141-2331-1, 82141-2332-1, 82141-2333-1
82141-2334-1, 82141-2336-1 & 82141-2338-1
Water, Butylene Glycol Cocoate,Hexylene Glycol, C12-15
Alkyl Benzoate, Cetearyl Alcohol, Butyloctyl Salicylate,
Styrene/Acrylates Copolymer, Dicaprylyl Carbonate, Coco
Glucoside, Myristyl Alcohol, Ceteareth-25, Propylene Glycol,
Dipropylene Glycol Dibenzoate, Phenoxyethanol, Silica,
Disodium Ethylene Dicocamide PEG-15 Disulfate,
Phenylpropanol, Myristyl Glucoside, Panthenol, Calaguala
( Polypodium leucotomos) Leaf Extract, Sodium Hyaluronate,
Caprylyl Glycol, Mica, Tea ( Camellia sinensis) Leaf Extract,
Propanediol, PPG-15 Stearyl Ether Benzoate,
Stearalkonium Hectorite, Propylene Carbonate, Tocopheryl
Acetate, BHT, Citric Acid, Disodium EDTA, Fragrance,
Linalool, Synthetic Iron Oxide* (CI 77491, CI 77492, CI
77499), Titanium Dioxide* (CI 77891), Bismuth Oxychloride*
(CI 77163).
* Contains one or more of these ingredients.
NDCs 82141-2335-1
Water, Butylene Glycol Cocoate,Hexylene Glycol, C12-15
Alkyl Benzoate, Cetearyl Alcohol, Butyloctyl Salicylate,
Styrene/Acrylates Copolymer, Dicaprylyl Carbonate, Coco
Glucoside, Myristyl Alcohol, Ceteareth-25, Propylene Glycol,
Dipropylene Glycol Dibenzoate, Phenoxyethanol, Silica,
Disodium Ethylene Dicocamide PEG-15 Disulfate,
Phenylpropanol, Myristyl Glucoside, Panthenol, Calaguala
( Polypodium leucotomos) Leaf Extract, Sodium Hyaluronate,
Caprylyl Glycol, Mica, Tea ( Camellia sinensis) Leaf Extract,
Propanediol, PPG-15 Stearyl Ether Benzoate,
Stearalkonium Hectorite, Propylene Carbonate, Tocopheryl
Acetate, BHT, Citric Acid, Disodium EDTA,
Triethoxycaprylylsilane, Fragrance, Linalool, Synthetic Iron
Oxide* (CI 77491, CI 77492, CI 77499), Titanium Dioxide*
(CI 77891), Bismuth Oxychloride* (CI 77163).
* Contains one or more of these ingredients.
NDCs 82141-2337-1 &82141-2339-1
Water, Butylene Glycol Cocoate,Hexylene Glycol, C12-15
Alkyl Benzoate, Butyloctyl Salicylate, Styrene/Acrylates
Copolymer, Ceteareth-25, Glyceril Stearate, PEG-100
Stearate, Propylene Glycol, Dipropylene Glycol Dibenzoate,
Silica, Phenoxyethanol, Disodium Ethylene Dicocamide
PEG-15 Disulfate, Phenylpropanol, Panthenol, Calaguala
( Polypodium leucotomos) Leaf Extract, Sodium Hyaluronate,
Caprylyl Glycol, Mica, Tea ( Camellia sinensis) Leaf Extract,
Propanediol, PPG-15 Stearyl Ether Benzoate, Tocopheryl
Acetate, Steareth-21, Steareth-2, Triethoxycaprylylsilane,
BHT, Citric Acid, Disodium EDTA, Fragrance, Linalool,
Synthetic Iron Oxide* (CI 77491, CI 77492, CI 77499).
* Contains one or more of these ingredients.
NDCs 82141-2340-1 & 82141-2341-1
Water, Butylene Glycol Cocoate,Hexylene Glycol, C12-15
Alkyl Benzoate, Butyloctyl Salicylate, Styrene/Acrylates
Copolymer, Ceteareth-25, Propylene Glycol, Dipropylene
Glycol Dibenzoate, Silica, Phenoxyethanol, Phenylpropanol,
Disodium Ethylene Dicocamide PEG-15 Disulfate,
Panthenol, Calaguala ( Polypodium leucotomos) Leaf
Extract, Sodium Hyaluronate, Caprylyl Glycol, Mica, Tea
( Camellia sinensis) Leaf Extract, Propanediol, PPG-15
Stearyl Ether Benzoate, Tocopheryl Acetate, Glyceryl
Stearate, PEG-100 Stearate, Steareth-21, Steareth-2,
Triethoxycaprylylsilane, BHT, Citric Acid, Disodium EDTA,
Fragrance, Linalool, Synthetic Iron Oxide* (CI 77491, CI
77492, CI 77499).
* Contains one or more of these ingredients.
- toty Ilumina CC Cream 1N
- toty Ilumina CC Cream 1W
- toty Ilumina CC Cream 1C
- toty Ilumina CC Cream 2N
- toty Ilumina CC Cream 2W
- toty Ilumina CC Cream 2C
- toty Ilumina CC Cream 3N
- toty Ilumina CC Cream 3W
- toty Ilumina CC Cream 3C
- toty Ilumina CC Cream 4N
- toty Ilumina CC Cream 4W
- toty Ilumina CC Cream 4C
- toty Ilumina CC Cream 5W
- toty Ilumina CC Cream 5W1
- toty Ilumina CC Cream 5W2
-
INGREDIENTS AND APPEARANCE
TOTY ILUMINA CC CREAM 1N
titanium dioxide, zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82141-2327 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 9 g in 100 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 6.3 g in 100 g Inactive Ingredients Ingredient Name Strength 1,3-BUTYLENE GLYCOL COCOATE (UNII: B2JUC5N9EH) BUTYL METHACRYLATE/METHYL METHACRYLATE/METHACRYLIC ACID/STYRENE CROSSPOLYMER (UNII: V5RS026Q0H) CAPRYLYL GLYCOL (UNII: 00YIU5438U) GREEN TEA LEAF (UNII: W2ZU1RY8B0) STEARALKONIUM HECTORITE (UNII: OLX698AH5P) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PHLEBODIUM AUREUM LEAF (UNII: BG58BW230G) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) COCO GLUCOSIDE (UNII: ICS790225B) WATER (UNII: 059QF0KO0R) HEXYLENE GLYCOL (UNII: KEH0A3F75J) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) CETEARETH-25 (UNII: 8FA93U5T67) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) LINALOOL, (+/-)- (UNII: D81QY6I88E) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) HYALURONATE SODIUM (UNII: YSE9PPT4TH) DICAPRYLYL CARBONATE (UNII: 609A3V1SUA) DIPROPYLENE GLYCOL DIBENZOATE (UNII: 6OA5ZDY41O) PROPANEDIOL (UNII: 5965N8W85T) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) PHENOXYETHANOL (UNII: HIE492ZZ3T) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) DISODIUM ETHYLENE DICOCAMIDE PEG-15 DISULFATE (UNII: QI9A6U005W) PPG-15 STEARYL ETHER BENZOATE (UNII: 80D2J6361M) PHENYLPROPANOL (UNII: 0F897O3O4M) MYRISTYL ALCOHOL (UNII: V42034O9PU) MYRISTYL GLUCOSIDE (UNII: 6AK28695LF) PANTHENOL (UNII: WV9CM0O67Z) PROPYLENE CARBONATE (UNII: 8D08K3S51E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82141-2327-1 1 in 1 CARTON 10/01/2022 1 40 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final M020 10/01/2022 TOTY ILUMINA CC CREAM 4C
titanium dioxide, zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82141-2338 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 9 g in 100 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 6.3 g in 100 g Inactive Ingredients Ingredient Name Strength BUTYL METHACRYLATE/METHYL METHACRYLATE/METHACRYLIC ACID/STYRENE CROSSPOLYMER (UNII: V5RS026Q0H) 1,3-BUTYLENE GLYCOL COCOATE (UNII: B2JUC5N9EH) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CAPRYLYL GLYCOL (UNII: 00YIU5438U) GREEN TEA LEAF (UNII: W2ZU1RY8B0) STEARALKONIUM HECTORITE (UNII: OLX698AH5P) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PHLEBODIUM AUREUM LEAF (UNII: BG58BW230G) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) COCO GLUCOSIDE (UNII: ICS790225B) WATER (UNII: 059QF0KO0R) HEXYLENE GLYCOL (UNII: KEH0A3F75J) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) CETEARETH-25 (UNII: 8FA93U5T67) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) LINALOOL, (+/-)- (UNII: D81QY6I88E) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) HYALURONATE SODIUM (UNII: YSE9PPT4TH) DICAPRYLYL CARBONATE (UNII: 609A3V1SUA) DIPROPYLENE GLYCOL DIBENZOATE (UNII: 6OA5ZDY41O) PROPANEDIOL (UNII: 5965N8W85T) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) PHENOXYETHANOL (UNII: HIE492ZZ3T) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) DISODIUM ETHYLENE DICOCAMIDE PEG-15 DISULFATE (UNII: QI9A6U005W) PPG-15 STEARYL ETHER BENZOATE (UNII: 80D2J6361M) PHENYLPROPANOL (UNII: 0F897O3O4M) MYRISTYL ALCOHOL (UNII: V42034O9PU) MYRISTYL GLUCOSIDE (UNII: 6AK28695LF) PANTHENOL (UNII: WV9CM0O67Z) PROPYLENE CARBONATE (UNII: 8D08K3S51E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82141-2338-1 1 in 1 CARTON 10/01/2022 1 40 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final M020 10/01/2022 TOTY ILUMINA CC CREAM 1C
titanium dioxide, zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82141-2329 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 9 g in 100 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 6.3 g in 100 g Inactive Ingredients Ingredient Name Strength BUTYL METHACRYLATE/METHYL METHACRYLATE/METHACRYLIC ACID/STYRENE CROSSPOLYMER (UNII: V5RS026Q0H) 1,3-BUTYLENE GLYCOL COCOATE (UNII: B2JUC5N9EH) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CAPRYLYL GLYCOL (UNII: 00YIU5438U) GREEN TEA LEAF (UNII: W2ZU1RY8B0) STEARALKONIUM HECTORITE (UNII: OLX698AH5P) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PHLEBODIUM AUREUM LEAF (UNII: BG58BW230G) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) COCO GLUCOSIDE (UNII: ICS790225B) WATER (UNII: 059QF0KO0R) HEXYLENE GLYCOL (UNII: KEH0A3F75J) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) CETEARETH-25 (UNII: 8FA93U5T67) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) LINALOOL, (+/-)- (UNII: D81QY6I88E) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) HYALURONATE SODIUM (UNII: YSE9PPT4TH) DICAPRYLYL CARBONATE (UNII: 609A3V1SUA) DIPROPYLENE GLYCOL DIBENZOATE (UNII: 6OA5ZDY41O) PROPANEDIOL (UNII: 5965N8W85T) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) PHENOXYETHANOL (UNII: HIE492ZZ3T) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) DISODIUM ETHYLENE DICOCAMIDE PEG-15 DISULFATE (UNII: QI9A6U005W) PPG-15 STEARYL ETHER BENZOATE (UNII: 80D2J6361M) PHENYLPROPANOL (UNII: 0F897O3O4M) MYRISTYL ALCOHOL (UNII: V42034O9PU) MYRISTYL GLUCOSIDE (UNII: 6AK28695LF) PANTHENOL (UNII: WV9CM0O67Z) PROPYLENE CARBONATE (UNII: 8D08K3S51E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82141-2329-1 1 in 1 CARTON 10/01/2022 1 40 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final M020 10/01/2022 TOTY ILUMINA CC CREAM 1W
titanium dioxide, zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82141-2328 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 9 g in 100 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 6.3 g in 100 g Inactive Ingredients Ingredient Name Strength BUTYL METHACRYLATE/METHYL METHACRYLATE/METHACRYLIC ACID/STYRENE CROSSPOLYMER (UNII: V5RS026Q0H) 1,3-BUTYLENE GLYCOL COCOATE (UNII: B2JUC5N9EH) CAPRYLYL GLYCOL (UNII: 00YIU5438U) GREEN TEA LEAF (UNII: W2ZU1RY8B0) STEARALKONIUM HECTORITE (UNII: OLX698AH5P) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PHLEBODIUM AUREUM LEAF (UNII: BG58BW230G) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) COCO GLUCOSIDE (UNII: ICS790225B) WATER (UNII: 059QF0KO0R) HEXYLENE GLYCOL (UNII: KEH0A3F75J) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) CETEARETH-25 (UNII: 8FA93U5T67) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) LINALOOL, (+/-)- (UNII: D81QY6I88E) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) HYALURONATE SODIUM (UNII: YSE9PPT4TH) DICAPRYLYL CARBONATE (UNII: 609A3V1SUA) DIPROPYLENE GLYCOL DIBENZOATE (UNII: 6OA5ZDY41O) PROPANEDIOL (UNII: 5965N8W85T) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) PHENOXYETHANOL (UNII: HIE492ZZ3T) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) DISODIUM ETHYLENE DICOCAMIDE PEG-15 DISULFATE (UNII: QI9A6U005W) PPG-15 STEARYL ETHER BENZOATE (UNII: 80D2J6361M) PHENYLPROPANOL (UNII: 0F897O3O4M) MYRISTYL ALCOHOL (UNII: V42034O9PU) MYRISTYL GLUCOSIDE (UNII: 6AK28695LF) PANTHENOL (UNII: WV9CM0O67Z) PROPYLENE CARBONATE (UNII: 8D08K3S51E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82141-2328-1 1 in 1 CARTON 10/01/2022 1 40 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final M020 10/01/2022 TOTY ILUMINA CC CREAM 2N
titanium dioxide, zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82141-2330 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 9 g in 100 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 6.3 g in 100 g Inactive Ingredients Ingredient Name Strength BUTYL METHACRYLATE/METHYL METHACRYLATE/METHACRYLIC ACID/STYRENE CROSSPOLYMER (UNII: V5RS026Q0H) 1,3-BUTYLENE GLYCOL COCOATE (UNII: B2JUC5N9EH) CAPRYLYL GLYCOL (UNII: 00YIU5438U) GREEN TEA LEAF (UNII: W2ZU1RY8B0) STEARALKONIUM HECTORITE (UNII: OLX698AH5P) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PHLEBODIUM AUREUM LEAF (UNII: BG58BW230G) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) COCO GLUCOSIDE (UNII: ICS790225B) WATER (UNII: 059QF0KO0R) HEXYLENE GLYCOL (UNII: KEH0A3F75J) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) CETEARETH-25 (UNII: 8FA93U5T67) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) LINALOOL, (+/-)- (UNII: D81QY6I88E) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) HYALURONATE SODIUM (UNII: YSE9PPT4TH) DICAPRYLYL CARBONATE (UNII: 609A3V1SUA) DIPROPYLENE GLYCOL DIBENZOATE (UNII: 6OA5ZDY41O) PROPANEDIOL (UNII: 5965N8W85T) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) PHENOXYETHANOL (UNII: HIE492ZZ3T) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) DISODIUM ETHYLENE DICOCAMIDE PEG-15 DISULFATE (UNII: QI9A6U005W) PPG-15 STEARYL ETHER BENZOATE (UNII: 80D2J6361M) PHENYLPROPANOL (UNII: 0F897O3O4M) MYRISTYL ALCOHOL (UNII: V42034O9PU) MYRISTYL GLUCOSIDE (UNII: 6AK28695LF) PANTHENOL (UNII: WV9CM0O67Z) PROPYLENE CARBONATE (UNII: 8D08K3S51E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82141-2330-1 1 in 1 CARTON 10/01/2022 1 40 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final M020 10/01/2022 TOTY ILUMINA CC CREAM 3C
titanium dioxide, zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82141-2335 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 9 g in 100 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 6.3 g in 100 g Inactive Ingredients Ingredient Name Strength 1,3-BUTYLENE GLYCOL COCOATE (UNII: B2JUC5N9EH) BUTYL METHACRYLATE/METHYL METHACRYLATE/METHACRYLIC ACID/STYRENE CROSSPOLYMER (UNII: V5RS026Q0H) BISMUTH OXYCHLORIDE (UNII: 4ZR792I587) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CAPRYLYL GLYCOL (UNII: 00YIU5438U) GREEN TEA LEAF (UNII: W2ZU1RY8B0) STEARALKONIUM HECTORITE (UNII: OLX698AH5P) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PHLEBODIUM AUREUM LEAF (UNII: BG58BW230G) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) COCO GLUCOSIDE (UNII: ICS790225B) WATER (UNII: 059QF0KO0R) HEXYLENE GLYCOL (UNII: KEH0A3F75J) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) CETEARETH-25 (UNII: 8FA93U5T67) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) LINALOOL, (+/-)- (UNII: D81QY6I88E) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) HYALURONATE SODIUM (UNII: YSE9PPT4TH) DICAPRYLYL CARBONATE (UNII: 609A3V1SUA) DIPROPYLENE GLYCOL DIBENZOATE (UNII: 6OA5ZDY41O) PROPANEDIOL (UNII: 5965N8W85T) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) PHENOXYETHANOL (UNII: HIE492ZZ3T) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) DISODIUM ETHYLENE DICOCAMIDE PEG-15 DISULFATE (UNII: QI9A6U005W) PPG-15 STEARYL ETHER BENZOATE (UNII: 80D2J6361M) PHENYLPROPANOL (UNII: 0F897O3O4M) MYRISTYL ALCOHOL (UNII: V42034O9PU) MYRISTYL GLUCOSIDE (UNII: 6AK28695LF) PANTHENOL (UNII: WV9CM0O67Z) PROPYLENE CARBONATE (UNII: 8D08K3S51E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82141-2335-1 1 in 1 CARTON 10/01/2022 1 40 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final M020 10/01/2022 TOTY ILUMINA CC CREAM 5W1
titanium dioxide, zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82141-2340 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 9 g in 100 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 6.3 g in 100 g Inactive Ingredients Ingredient Name Strength BUTYL METHACRYLATE/METHYL METHACRYLATE/METHACRYLIC ACID/STYRENE CROSSPOLYMER (UNII: V5RS026Q0H) 1,3-BUTYLENE GLYCOL COCOATE (UNII: B2JUC5N9EH) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) PEG-100 STEARATE (UNII: YD01N1999R) MICA (UNII: V8A1AW0880) STEARETH-21 (UNII: 53J3F32P58) STEARETH-2 (UNII: V56DFE46J5) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CAPRYLYL GLYCOL (UNII: 00YIU5438U) GREEN TEA LEAF (UNII: W2ZU1RY8B0) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PHLEBODIUM AUREUM LEAF (UNII: BG58BW230G) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) HEXYLENE GLYCOL (UNII: KEH0A3F75J) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) CETEARETH-25 (UNII: 8FA93U5T67) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) LINALOOL, (+/-)- (UNII: D81QY6I88E) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) HYALURONATE SODIUM (UNII: YSE9PPT4TH) DIPROPYLENE GLYCOL DIBENZOATE (UNII: 6OA5ZDY41O) PROPANEDIOL (UNII: 5965N8W85T) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) PHENOXYETHANOL (UNII: HIE492ZZ3T) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) DISODIUM ETHYLENE DICOCAMIDE PEG-15 DISULFATE (UNII: QI9A6U005W) PPG-15 STEARYL ETHER BENZOATE (UNII: 80D2J6361M) PHENYLPROPANOL (UNII: 0F897O3O4M) PANTHENOL (UNII: WV9CM0O67Z) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82141-2340-1 1 in 1 CARTON 10/01/2022 1 40 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final M020 10/01/2022 TOTY ILUMINA CC CREAM 5W2
titanium dioxide, zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82141-2341 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 9 g in 100 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 6.3 g in 100 g Inactive Ingredients Ingredient Name Strength BUTYL METHACRYLATE/METHYL METHACRYLATE/METHACRYLIC ACID/STYRENE CROSSPOLYMER (UNII: V5RS026Q0H) 1,3-BUTYLENE GLYCOL COCOATE (UNII: B2JUC5N9EH) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) PEG-100 STEARATE (UNII: YD01N1999R) MICA (UNII: V8A1AW0880) STEARETH-21 (UNII: 53J3F32P58) STEARETH-2 (UNII: V56DFE46J5) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CAPRYLYL GLYCOL (UNII: 00YIU5438U) GREEN TEA LEAF (UNII: W2ZU1RY8B0) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PHLEBODIUM AUREUM LEAF (UNII: BG58BW230G) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) HEXYLENE GLYCOL (UNII: KEH0A3F75J) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) CETEARETH-25 (UNII: 8FA93U5T67) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) LINALOOL, (+/-)- (UNII: D81QY6I88E) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) HYALURONATE SODIUM (UNII: YSE9PPT4TH) DIPROPYLENE GLYCOL DIBENZOATE (UNII: 6OA5ZDY41O) PROPANEDIOL (UNII: 5965N8W85T) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) PHENOXYETHANOL (UNII: HIE492ZZ3T) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) DISODIUM ETHYLENE DICOCAMIDE PEG-15 DISULFATE (UNII: QI9A6U005W) PPG-15 STEARYL ETHER BENZOATE (UNII: 80D2J6361M) PHENYLPROPANOL (UNII: 0F897O3O4M) PANTHENOL (UNII: WV9CM0O67Z) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82141-2341-1 1 in 1 CARTON 10/01/2022 1 40 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final M020 10/01/2022 TOTY ILUMINA CC CREAM 3W
titanium dioxide, zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82141-2334 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 9 g in 100 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 6.3 g in 100 g Inactive Ingredients Ingredient Name Strength 1,3-BUTYLENE GLYCOL COCOATE (UNII: B2JUC5N9EH) BUTYL METHACRYLATE/METHYL METHACRYLATE/METHACRYLIC ACID/STYRENE CROSSPOLYMER (UNII: V5RS026Q0H) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CAPRYLYL GLYCOL (UNII: 00YIU5438U) GREEN TEA LEAF (UNII: W2ZU1RY8B0) STEARALKONIUM HECTORITE (UNII: OLX698AH5P) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PHLEBODIUM AUREUM LEAF (UNII: BG58BW230G) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) COCO GLUCOSIDE (UNII: ICS790225B) WATER (UNII: 059QF0KO0R) HEXYLENE GLYCOL (UNII: KEH0A3F75J) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) CETEARETH-25 (UNII: 8FA93U5T67) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) LINALOOL, (+/-)- (UNII: D81QY6I88E) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) HYALURONATE SODIUM (UNII: YSE9PPT4TH) DICAPRYLYL CARBONATE (UNII: 609A3V1SUA) DIPROPYLENE GLYCOL DIBENZOATE (UNII: 6OA5ZDY41O) PROPANEDIOL (UNII: 5965N8W85T) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) PHENOXYETHANOL (UNII: HIE492ZZ3T) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) DISODIUM ETHYLENE DICOCAMIDE PEG-15 DISULFATE (UNII: QI9A6U005W) PPG-15 STEARYL ETHER BENZOATE (UNII: 80D2J6361M) PHENYLPROPANOL (UNII: 0F897O3O4M) MYRISTYL ALCOHOL (UNII: V42034O9PU) MYRISTYL GLUCOSIDE (UNII: 6AK28695LF) PANTHENOL (UNII: WV9CM0O67Z) PROPYLENE CARBONATE (UNII: 8D08K3S51E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82141-2334-1 1 in 1 CARTON 10/01/2022 1 40 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final M020 10/01/2022 TOTY ILUMINA CC CREAM 4N
titanium dioxide, zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82141-2336 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 9 g in 100 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 6.3 g in 100 g Inactive Ingredients Ingredient Name Strength BUTYL METHACRYLATE/METHYL METHACRYLATE/METHACRYLIC ACID/STYRENE CROSSPOLYMER (UNII: V5RS026Q0H) 1,3-BUTYLENE GLYCOL COCOATE (UNII: B2JUC5N9EH) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CAPRYLYL GLYCOL (UNII: 00YIU5438U) GREEN TEA LEAF (UNII: W2ZU1RY8B0) STEARALKONIUM HECTORITE (UNII: OLX698AH5P) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PHLEBODIUM AUREUM LEAF (UNII: BG58BW230G) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) COCO GLUCOSIDE (UNII: ICS790225B) WATER (UNII: 059QF0KO0R) HEXYLENE GLYCOL (UNII: KEH0A3F75J) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) CETEARETH-25 (UNII: 8FA93U5T67) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) LINALOOL, (+/-)- (UNII: D81QY6I88E) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) HYALURONATE SODIUM (UNII: YSE9PPT4TH) DICAPRYLYL CARBONATE (UNII: 609A3V1SUA) DIPROPYLENE GLYCOL DIBENZOATE (UNII: 6OA5ZDY41O) PROPANEDIOL (UNII: 5965N8W85T) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) PHENOXYETHANOL (UNII: HIE492ZZ3T) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) DISODIUM ETHYLENE DICOCAMIDE PEG-15 DISULFATE (UNII: QI9A6U005W) PPG-15 STEARYL ETHER BENZOATE (UNII: 80D2J6361M) PHENYLPROPANOL (UNII: 0F897O3O4M) MYRISTYL ALCOHOL (UNII: V42034O9PU) MYRISTYL GLUCOSIDE (UNII: 6AK28695LF) PANTHENOL (UNII: WV9CM0O67Z) PROPYLENE CARBONATE (UNII: 8D08K3S51E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82141-2336-1 1 in 1 CARTON 10/01/2022 1 40 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final M020 10/01/2022 TOTY ILUMINA CC CREAM 5W
titanium dioxide, zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82141-2339 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 9 g in 100 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 6.3 g in 100 g Inactive Ingredients Ingredient Name Strength 1,3-BUTYLENE GLYCOL COCOATE (UNII: B2JUC5N9EH) BUTYL METHACRYLATE/METHYL METHACRYLATE/METHACRYLIC ACID/STYRENE CROSSPOLYMER (UNII: V5RS026Q0H) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) PEG-100 STEARATE (UNII: YD01N1999R) MICA (UNII: V8A1AW0880) STEARETH-21 (UNII: 53J3F32P58) STEARETH-2 (UNII: V56DFE46J5) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CAPRYLYL GLYCOL (UNII: 00YIU5438U) GREEN TEA LEAF (UNII: W2ZU1RY8B0) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PHLEBODIUM AUREUM LEAF (UNII: BG58BW230G) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) HEXYLENE GLYCOL (UNII: KEH0A3F75J) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) CETEARETH-25 (UNII: 8FA93U5T67) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) LINALOOL, (+/-)- (UNII: D81QY6I88E) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) HYALURONATE SODIUM (UNII: YSE9PPT4TH) DIPROPYLENE GLYCOL DIBENZOATE (UNII: 6OA5ZDY41O) PROPANEDIOL (UNII: 5965N8W85T) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) PHENOXYETHANOL (UNII: HIE492ZZ3T) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) DISODIUM ETHYLENE DICOCAMIDE PEG-15 DISULFATE (UNII: QI9A6U005W) PPG-15 STEARYL ETHER BENZOATE (UNII: 80D2J6361M) PHENYLPROPANOL (UNII: 0F897O3O4M) PANTHENOL (UNII: WV9CM0O67Z) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82141-2339-1 1 in 1 CARTON 10/01/2022 1 40 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final M020 10/01/2022 TOTY ILUMINA CC CREAM 3N
titanium dioxide, zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82141-2333 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 9 g in 100 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 6.3 g in 100 g Inactive Ingredients Ingredient Name Strength 1,3-BUTYLENE GLYCOL COCOATE (UNII: B2JUC5N9EH) BUTYL METHACRYLATE/METHYL METHACRYLATE/METHACRYLIC ACID/STYRENE CROSSPOLYMER (UNII: V5RS026Q0H) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CAPRYLYL GLYCOL (UNII: 00YIU5438U) GREEN TEA LEAF (UNII: W2ZU1RY8B0) STEARALKONIUM HECTORITE (UNII: OLX698AH5P) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PHLEBODIUM AUREUM LEAF (UNII: BG58BW230G) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) COCO GLUCOSIDE (UNII: ICS790225B) WATER (UNII: 059QF0KO0R) HEXYLENE GLYCOL (UNII: KEH0A3F75J) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) CETEARETH-25 (UNII: 8FA93U5T67) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) LINALOOL, (+/-)- (UNII: D81QY6I88E) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) HYALURONATE SODIUM (UNII: YSE9PPT4TH) DICAPRYLYL CARBONATE (UNII: 609A3V1SUA) DIPROPYLENE GLYCOL DIBENZOATE (UNII: 6OA5ZDY41O) PROPANEDIOL (UNII: 5965N8W85T) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) PHENOXYETHANOL (UNII: HIE492ZZ3T) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) DISODIUM ETHYLENE DICOCAMIDE PEG-15 DISULFATE (UNII: QI9A6U005W) PPG-15 STEARYL ETHER BENZOATE (UNII: 80D2J6361M) PHENYLPROPANOL (UNII: 0F897O3O4M) MYRISTYL ALCOHOL (UNII: V42034O9PU) MYRISTYL GLUCOSIDE (UNII: 6AK28695LF) PANTHENOL (UNII: WV9CM0O67Z) PROPYLENE CARBONATE (UNII: 8D08K3S51E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82141-2333-1 1 in 1 CARTON 10/01/2022 1 40 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final M020 10/01/2022 TOTY ILUMINA CC CREAM 4W
titanium dioxide, zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82141-2337 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 9 g in 100 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 6.3 g in 100 g Inactive Ingredients Ingredient Name Strength BUTYL METHACRYLATE/METHYL METHACRYLATE/METHACRYLIC ACID/STYRENE CROSSPOLYMER (UNII: V5RS026Q0H) 1,3-BUTYLENE GLYCOL COCOATE (UNII: B2JUC5N9EH) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) PEG-100 STEARATE (UNII: YD01N1999R) MICA (UNII: V8A1AW0880) STEARETH-21 (UNII: 53J3F32P58) STEARETH-2 (UNII: V56DFE46J5) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CAPRYLYL GLYCOL (UNII: 00YIU5438U) GREEN TEA LEAF (UNII: W2ZU1RY8B0) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PHLEBODIUM AUREUM LEAF (UNII: BG58BW230G) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) HEXYLENE GLYCOL (UNII: KEH0A3F75J) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) CETEARETH-25 (UNII: 8FA93U5T67) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) LINALOOL, (+/-)- (UNII: D81QY6I88E) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) HYALURONATE SODIUM (UNII: YSE9PPT4TH) DIPROPYLENE GLYCOL DIBENZOATE (UNII: 6OA5ZDY41O) PROPANEDIOL (UNII: 5965N8W85T) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) PHENOXYETHANOL (UNII: HIE492ZZ3T) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) DISODIUM ETHYLENE DICOCAMIDE PEG-15 DISULFATE (UNII: QI9A6U005W) PPG-15 STEARYL ETHER BENZOATE (UNII: 80D2J6361M) PHENYLPROPANOL (UNII: 0F897O3O4M) PANTHENOL (UNII: WV9CM0O67Z) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82141-2337-1 1 in 1 CARTON 10/01/2022 1 40 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final M020 10/01/2022 TOTY ILUMINA CC CREAM 2C
titanium dioxide, zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82141-2332 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 9 g in 100 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 6.3 g in 100 g Inactive Ingredients Ingredient Name Strength 1,3-BUTYLENE GLYCOL COCOATE (UNII: B2JUC5N9EH) BUTYL METHACRYLATE/METHYL METHACRYLATE/METHACRYLIC ACID/STYRENE CROSSPOLYMER (UNII: V5RS026Q0H) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CAPRYLYL GLYCOL (UNII: 00YIU5438U) GREEN TEA LEAF (UNII: W2ZU1RY8B0) STEARALKONIUM HECTORITE (UNII: OLX698AH5P) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PHLEBODIUM AUREUM LEAF (UNII: BG58BW230G) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) COCO GLUCOSIDE (UNII: ICS790225B) WATER (UNII: 059QF0KO0R) HEXYLENE GLYCOL (UNII: KEH0A3F75J) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) CETEARETH-25 (UNII: 8FA93U5T67) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) LINALOOL, (+/-)- (UNII: D81QY6I88E) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) HYALURONATE SODIUM (UNII: YSE9PPT4TH) DICAPRYLYL CARBONATE (UNII: 609A3V1SUA) DIPROPYLENE GLYCOL DIBENZOATE (UNII: 6OA5ZDY41O) PROPANEDIOL (UNII: 5965N8W85T) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) PHENOXYETHANOL (UNII: HIE492ZZ3T) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) DISODIUM ETHYLENE DICOCAMIDE PEG-15 DISULFATE (UNII: QI9A6U005W) PPG-15 STEARYL ETHER BENZOATE (UNII: 80D2J6361M) PHENYLPROPANOL (UNII: 0F897O3O4M) MYRISTYL ALCOHOL (UNII: V42034O9PU) MYRISTYL GLUCOSIDE (UNII: 6AK28695LF) PANTHENOL (UNII: WV9CM0O67Z) PROPYLENE CARBONATE (UNII: 8D08K3S51E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82141-2332-1 1 in 1 CARTON 10/01/2022 1 40 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final M020 10/01/2022 TOTY ILUMINA CC CREAM 2W
titanium dioxide, zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82141-2331 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 9 g in 100 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 6.3 g in 100 g Inactive Ingredients Ingredient Name Strength BUTYL METHACRYLATE/METHYL METHACRYLATE/METHACRYLIC ACID/STYRENE CROSSPOLYMER (UNII: V5RS026Q0H) 1,3-BUTYLENE GLYCOL COCOATE (UNII: B2JUC5N9EH) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CAPRYLYL GLYCOL (UNII: 00YIU5438U) GREEN TEA LEAF (UNII: W2ZU1RY8B0) STEARALKONIUM HECTORITE (UNII: OLX698AH5P) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PHLEBODIUM AUREUM LEAF (UNII: BG58BW230G) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) COCO GLUCOSIDE (UNII: ICS790225B) WATER (UNII: 059QF0KO0R) HEXYLENE GLYCOL (UNII: KEH0A3F75J) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) CETEARETH-25 (UNII: 8FA93U5T67) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) LINALOOL, (+/-)- (UNII: D81QY6I88E) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) HYALURONATE SODIUM (UNII: YSE9PPT4TH) DICAPRYLYL CARBONATE (UNII: 609A3V1SUA) DIPROPYLENE GLYCOL DIBENZOATE (UNII: 6OA5ZDY41O) PROPANEDIOL (UNII: 5965N8W85T) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) PHENOXYETHANOL (UNII: HIE492ZZ3T) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) DISODIUM ETHYLENE DICOCAMIDE PEG-15 DISULFATE (UNII: QI9A6U005W) PPG-15 STEARYL ETHER BENZOATE (UNII: 80D2J6361M) PHENYLPROPANOL (UNII: 0F897O3O4M) MYRISTYL ALCOHOL (UNII: V42034O9PU) MYRISTYL GLUCOSIDE (UNII: 6AK28695LF) PANTHENOL (UNII: WV9CM0O67Z) PROPYLENE CARBONATE (UNII: 8D08K3S51E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82141-2331-1 1 in 1 CARTON 10/01/2022 1 40 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final M020 10/01/2022 Labeler - Chipican LLC (118132015) Establishment Name Address ID/FEI Business Operations Industrial Farmaceutica Cantabria SA 470471158 manufacture(82141-2327, 82141-2328, 82141-2329, 82141-2330, 82141-2331, 82141-2332, 82141-2333, 82141-2334, 82141-2335, 82141-2336, 82141-2337, 82141-2338, 82141-2339, 82141-2340, 82141-2341) , pack(82141-2327, 82141-2328, 82141-2329, 82141-2330, 82141-2331, 82141-2332, 82141-2333, 82141-2334, 82141-2335, 82141-2336, 82141-2337, 82141-2338, 82141-2339, 82141-2340, 82141-2341) , label(82141-2327, 82141-2328, 82141-2329, 82141-2330, 82141-2331, 82141-2332, 82141-2333, 82141-2334, 82141-2335, 82141-2336, 82141-2337, 82141-2338, 82141-2339, 82141-2340, 82141-2341)