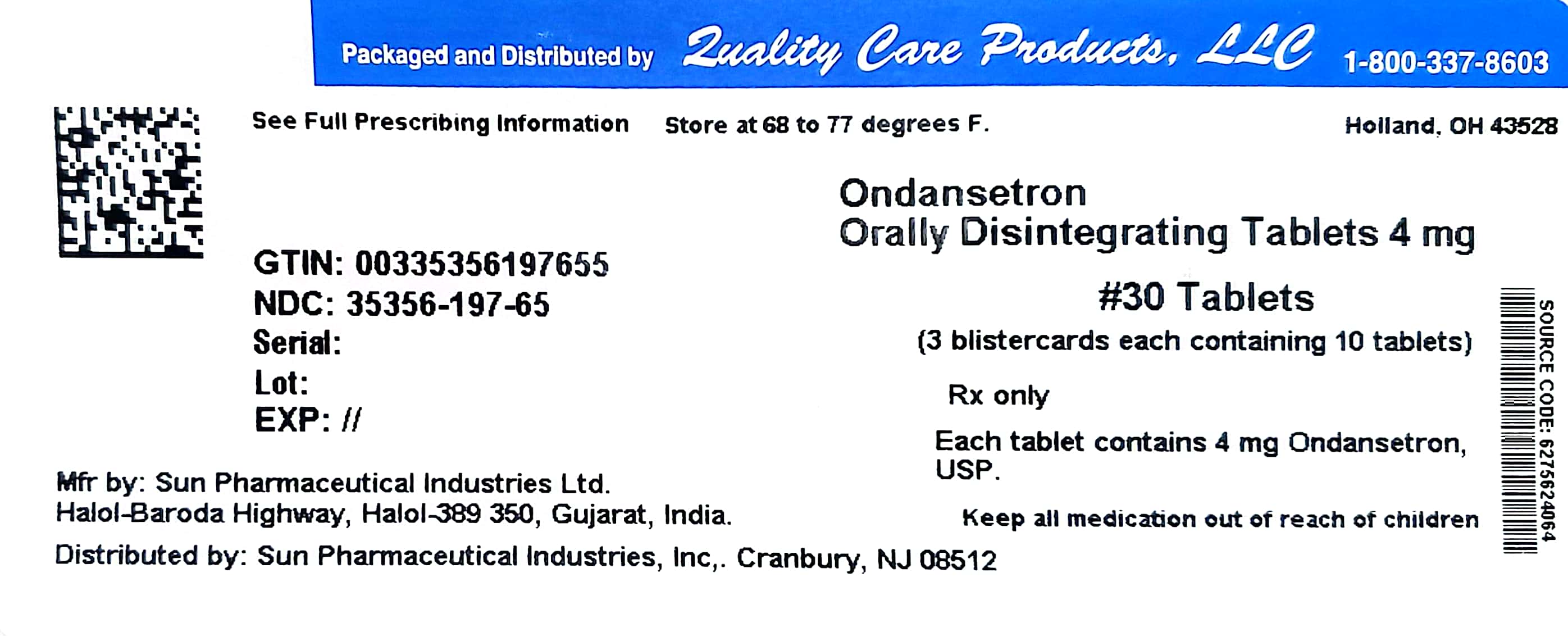
Label: ONDANSETRON tablet, orally disintegrating
- NDC Code(s): 35356-197-65
- Packager: Quality Care Products LLC
- This is a repackaged label.
- Source NDC Code(s): 62756-240
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated February 14, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
DESCRIPTIONThe active ingredient in ondansetron orally disintegrating tablets is ondansetron base, the racemic form of ondansetron, and a selective blocking agent of the serotonin 5-HT3 receptor type. Chemically it is (±)1, 2, 3, 9-tetrahydro-9-methyl-3-[(2-methyl-1H-imidazol-1-yl)methyl]-4H-carbazol-4-one. It has the following structural formula
The empirical formula is C18H19N3O representing a molecular weight of 293.4.
Each 4 mg ondansetron orally disintegrating tablets for oral administration contains 4 mg ondansetron base. Each 8 mg ondansetron orally disintegrating tablets for oral administration contains 8 mg ondansetron base. Each ondansetron orally disintegrating tablet also contains the inactive ingredients aspartame, colloidal silicon dioxide, croscarmellose sodium, glycerol distearate, magnesium stearate, mannitol, talc and strawberry flavor. Ondansetron orally disintegrating tablets are orally administered formulation of ondansetron which rapidly disintegrates on the tongue and does not require water to aid dissolution or swallowing.
Does not meet USP Disintegration Time. This product disintegrates in approximately 60 seconds.
-
CLINICAL PHARMACOLOGY
CLINICAL PHARMACOLOGY
Pharmacodynamics
Ondansetron is a selective 5-HT3 receptor antagonist. While its mechanism of action has not been fully characterized, ondansetron is not a dopamine-receptor antagonist. Serotonin receptors of the 5-HT3 type are present both peripherally on vagal nerve terminals and centrally in the chemoreceptor trigger zone of the area postrema. It is not certain whether ondansetron’s antiemetic action is mediated centrally, peripherally, or in both sites. However, cytotoxic chemotherapy appears to be associated with release of serotonin from the enterochromaffin cells of the small intestine. In humans, urinary 5-HIAA (5-hydroxyindoleacetic acid) excretion increases after cisplatin administration in parallel with the onset of emesis. The released serotonin may stimulate the vagal afferents through the 5-HT3 receptors and initiate the vomiting reflex.
In animals, the emetic response to cisplatin can be prevented by pretreatment with an inhibitor of serotonin synthesis, bilateral abdominal vagotomy and greater splanchnic nerve section, or pretreatment with a serotonin 5-HT3 receptor antagonist.
In normal volunteers, single intravenous doses of 0.15 mg/kg of ondansetron had no effect on esophageal motility, gastric motility, lower esophageal sphincter pressure, or small intestinal transit time. Multiday administration of ondansetron has been shown to slow colonic transit in normal volunteers. Ondansetron has no effect on plasma prolactin concentrations.
Ondansetron does not alter the respiratory depressant effects produced by alfentanil or the degree of neuromuscular blockade produced by atracurium. Interactions with general or local anesthetics have not been studied.
Pharmacokinetics
Ondansetron is well absorbed from the gastrointestinal tract and undergoes some first-pass metabolism. Mean bioavailability in healthy subjects, following administration of a single 8 mg tablet, is approximately 56%.
Ondansetron systemic exposure does not increase proportionately to dose. AUC from a 16 mg tablet was 24% greater than predicted from an 8 mg tablet dose. This may reflect some reduction of first-pass metabolism at higher oral doses. Bioavailability is also slightly enhanced by the presence of food but unaffected by antacids.
Ondansetron is extensively metabolized in humans, with approximately 5% of a radiolabeled dose recovered as the parent compound from the urine. The primary metabolic pathway is hydroxylation on the indole ring followed by subsequent glucuronide or sulfate conjugation. Although some nonconjugated metabolites have pharmacologic activity, these are not found in plasma at concentrations likely to significantly contribute to the biological activity of ondansetron.
In vitro metabolism studies have shown that ondansetron is a substrate for human hepatic cytochrome P-450 enzymes, including CYP1A2, CYP2D6, and CYP3A4. In terms of overall ondansetron turnover, CYP3A4 played the predominant role. Because of the multiplicity of metabolic enzymes capable of metabolizing ondansetron, it is likely that inhibition or loss of one enzyme (e.g., CYP2D6 genetic deficiency) will be compensated by others and may result in little change in overall rates of ondansetron elimination. Ondansetron elimination may be affected by cytochrome P-450 inducers. In a pharmacokinetic study of 16 epileptic patients maintained chronically on CYP3A4 inducers, carbamazepine, or phenytoin, reduction in AUC, Cmax, and T½ of ondansetron was observed.1 This resulted in a significant increase in clearance. However, on the basis of available data, no dosage adjustment for ondansetron is recommended (see PRECAUTIONS: Drug Interactions).
In humans, carmustine, etoposide, and cisplatin do not affect the pharmacokinetics of ondansetron.
Gender differences were shown in the disposition of ondansetron given as a single dose. The extent and rate of ondansetron's absorption is greater in women than men. Slower clearance in women, a smaller apparent volume of distribution (adjusted for weight), and higher absolute bioavailability resulted in higher plasma ondansetron levels. These higher plasma levels may in part be explained by differences in body weight between men and women. It is not known whether these gender-related differences were clinically important. More detailed pharmacokinetic information is contained in Tables 1 and 2 taken from 2 studies.
-
INDICATIONS & USAGE
INDICATIONS AND USAGE
1. Prevention of nausea and vomiting associated with highly emetogenic cancer chemotherapy, including cisplatin ≥50 mg/m2.
2. Prevention of nausea and vomiting associated with initial and repeat courses of moderately emetogenic cancer chemotherapy.
3. Prevention of nausea and vomiting associated with radiotherapy in patients receiving either total body irradiation, single high-dose fraction to the abdomen, or daily fractions to the abdomen.
4. Prevention of postoperative nausea and/or vomiting. As with other antiemetics, routine prophylaxis is not recommended for patients in whom there is little expectation that nausea and/or vomiting will occur postoperatively. In patients where nausea and/or vomiting must be avoided postoperatively, ondansetron orally disintegrating tablets are recommended even where the incidence of postoperative nausea and/or vomiting is low.
- CONTRAINDICATIONS
- WARNINGS
-
PRECAUTIONS
PRECAUTIONS
General
Ondansetron is not a drug that stimulates gastric or intestinal peristalsis. It should not be used instead of nasogastric suction. The use of ondansetron in patients following abdominal surgery or in patients with chemotherapy-induced nausea and vomiting may mask a progressive ileus and/or gastric distension.
Rarely and predominantly with intravenous ondansetron, transient ECG changes including QT interval prolongation have been reported.
-
ADVERSE REACTIONS
ADVERSE REACTIONS
The following have been reported as adverse events in clinical trials of patients treated with ondansetron. A causal relationship to therapy with ondansetron has been unclear in many cases.
Chemotherapy-Induced Nausea and VomitingThe adverse events in Table 5 have been reported in greater than or equal to 5% of adult patients receiving a single 24 mg ondansetron tablet in 2 trials. These patients were receiving concurrent highly emetogenic cisplatin-based chemotherapy regimens (cisplatin dose greater than or equal to 50 mg/m2).
Central Nervous System: There have been rare reports consistent with, but not diagnostic of, extrapyramidal reactions in patients receiving ondansetron.
Hepatic: In 723 patients receiving cyclophosphamide-based chemotherapy in US clinical trials, AST and/or ALT values have been reported to exceed twice the upper limit of normal in approximately 1% to 2% of patients receiving ondansetron tablets. The increases were transient and did not appear to be related to dose or duration of therapy. On repeat exposure, similar transient elevations in transaminase values occurred in some courses, but symptomatic hepatic disease did not occur. The role of cancer chemotherapy in these biochemical changes cannot be clearly determined.
There have been reports of liver failure and death in patients with cancer receiving concurrent medications including potentially hepatotoxic cytotoxic chemotherapy and antibiotics. The etiology of the liver failure is unclear.
Integumentary: Rash has occurred in approximately 1% of patients receiving ondansetron.
Other: Rare cases of anaphylaxis, bronchospasm, tachycardia, angina (chest pain), hypokalemia, electrocardiographic alterations, vascular occlusive events, and grand mal seizures have been reported. Except for bronchospasm and anaphylaxis, the relationship to ondansetron was unclear.
Radiation-Induced Nausea and VomitingThe adverse events reported in patients receiving ondansetron tablets and concurrent radiotherapy were similar to those reported in patients receiving ondansetron tablets and concurrent chemotherapy. The most frequently reported adverse events were headache, constipation, and diarrhea.
Postoperative Nausea and VomitingThe adverse events in Table 7 have been reported in greater than or equal to 5% of patients receiving ondansetron tablets at a dosage of 16 mg orally in clinical trials. With the exception of headache, rates of these events were not significantly different in the ondansetron and placebo groups. These patients were receiving multiple concomitant perioperative and postoperative medications
-
OVERDOSAGE
OVERDOSAGE
There is no specific antidote for ondansetron overdose. Patients should be managed with appropriate supportive therapy. Individual intravenous doses as large as 150 mg and total daily intravenous doses as large as 252 mg have been inadvertently administered without significant adverse events. These doses are more than 10 times the recommended daily dose.
In addition to the adverse events listed above, the following events have been described in the setting of ondansetron overdose: "Sudden blindness" (amaurosis) of 2 to 3 minutes' duration plus severe constipation occurred in 1 patient that was administered 72 mg of ondansetron intravenously as a single dose. Hypotension (and faintness) occurred in a patient that took 48 mg of ondansetron tablets. Following infusion of 32 mg over only a 4-minute period, a vasovagal episode with transient second-degree heart block was observed. In all instances, the events resolved completely.
-
DOSAGE & ADMINISTRATION
DOSAGE AND ADMINISTRATION
Instructions for Use/Handling Ondansetron Orally Disintegrating Tablets:Do not attempt to push ondansetron disintegrating tablets through the foil backing. With dry hands, PEEL BACK the foil backing of 1 blister and GENTLY remove the tablet. IMMEDIATELY place the ondansetron orally disintegrating tablet on top of the tongue where it will dissolve in seconds, then swallow with saliva. Administration with liquid is not necessary.
Prevention of Nausea and Vomiting Associated With Highly Emetogenic Cancer Chemotherapy:
The recommended adult oral dosage of ondansetron is 24 mg given as three 8 mg tablets administered 30 minutes before the start of single-day highly emetogenic chemotherapy, including cisplatin ≥50 mg/m2. Multiday, single-dose administration of a 24 mg dosage has not been studied.
Pediatric Use: There is no experience with the use of a 24 mg dosage in pediatric patients.
Geriatric Use: The dosage recommendation is the same as for the general population.
Prevention of Nausea and Vomiting Associated With Moderately Emetogenic Cancer Chemotherapy:The recommended adult oral dosage is one 8 mg ondansetron orally disintegrating tablet given twice a day. The first dose should be administered 30 minutes before the start of emetogenic chemotherapy, with a subsequent dose 8 hours after the first dose. One 8 mg ondansetron orally disintegrating tablet should be administered twice a day (every 12 hours) for 1 to 2 days after completion of chemotherapy.
Pediatric Use: For pediatric patients 12 years of age and older, the dosage is the same as for adults. For pediatric patients 4 through 11 years of age, the dosage is one 4 mg ondansetron orally disintegrating tablet given 3 times a day. The first dose should be administered 30 minutes before the start of emetogenic chemotherapy, with subsequent doses 4 and 8 hours after the first dose. One 4 mg ondansetron orally disintegrating tablet should be administered 3 times a day (every 8 hours) for 1 to 2 days after completion of chemotherapy
Geriatric Use: The dosage is the same as for the general population.
Prevention of Nausea and Vomiting Associated With Radiotherapy, Either Total Body Irradiation, or Single High-Dose Fraction or Daily Fractions to the Abdomen:The recommended oral dosage is one 8 mg ondansetron orally disintegrating tablets given 3 times a day.
For total body irradiation, one 8 mg ondansetron orally disintegrating tablet should be administered 1 to 2 hours before each fraction of radiotherapy administered each day.
For single high-dose fraction radiotherapy to the abdomen, one 8 mg ondansetron orally disintegrating tablet should be administered 1 to 2 hours before radiotherapy, with subsequent doses every 8 hours after the first dose for 1 to 2 days after completion of radiotherapy.
For daily fractionated radiotherapy to the abdomen, one 8 mg ondansetron orally disintegrating tablet should be administered 1 to 2 hours before radiotherapy, with subsequent doses every 8 hours after the first dose for each day radiotherapy is given.
Pediatric Use: There is no experience with the use of ondansetron orally disintegrating tablets, in the prevention of radiation-induced nausea and vomiting in pediatric patients.
Geriatric Use: The dosage recommendation is the same as for the general population.
Postoperative Nausea and Vomiting:
The recommended dosage is 16 mg given as two 8 mg ondansetron orally disintegrating tablets 1 hour before induction of anesthesia.
Pediatric Use: There is no experience with the use of ondansetron orally disintegrating tablets in the prevention of postoperative nausea and vomiting in pediatric patients.
Geriatric Use: The dosage is the same as for the general population.
Dosage Adjustment for Patients with Impaired Renal Function:The dosage recommendation is the same as for the general population. There is no experience beyond first-day administration of ondansetron.
Dosage Adjustment for Patients With Impaired Hepatic Function:
In patients with severe hepatic impairment (Child-Pugh2 score of 10 or greater), clearance is reduced and apparent volume of distribution is increased with a resultant increase in plasma half-life. In such patients, a total daily dose of 8 mg should not be exceeded.
-
HOW SUPPLIED
HOW SUPPLIED
Ondansetron orally disintegrating tablets, 4 mg (as 4 mg ondansetron base) are white to off white, oval, uncoated tablets debossed with "240" on one side, plain on the other side.
Bottle pack of 30 tablets with Child Resistant Cap -
Unit dose packs of 30 tablets (NDC 35356-197-65Ondansetron orally disintegrating tablets, 8 mg (as 8 mg ondansetron base) are white to off white, oval, uncoated tablets debossed with "241" on one side, plain on the other side.
Bottle pack of 30 tablets with Child Resistant Cap -
-
INFORMATION FOR PATIENTS
Information for Patients
Phenylketonurics: Phenylketonuric patients should be informed that ondansetron orally disintegrating tablets contain phenylalanine (a component of aspartame). Each 4 mg and 8 mg orally disintegrating tablet contains less than 0.04 mg phenylalanine.
Patients should be instructed not to remove ondansetron orally disintegrating tablets from the blister until just prior to dosing. The tablet should not be pushed through the foil. With dry hands, the blister backing should be peeled completely off the blister. The tablet should be gently removed and immediately placed on the tongue to dissolve and be swallowed with the saliva.
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ONDANSETRON
ondansetron tablet, orally disintegratingProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:35356-197(NDC:62756-240) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ONDANSETRON (UNII: 4AF302ESOS) (ONDANSETRON - UNII:4AF302ESOS) ONDANSETRON 4 mg Inactive Ingredients Ingredient Name Strength ASPARTAME (UNII: Z0H242BBR1) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) GLYCERYL DISTEARATE (UNII: 73071MW2KM) MAGNESIUM STEARATE (UNII: 70097M6I30) MANNITOL (UNII: 3OWL53L36A) TALC (UNII: 7SEV7J4R1U) Product Characteristics Color white (white to off white) Score no score Shape OVAL Size 10mm Flavor STRAWBERRY Imprint Code 240 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:35356-197-65 30 in 1 BLISTER PACK; Type 0: Not a Combination Product 11/10/2010 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA077557 11/10/2010 Labeler - Quality Care Products LLC (831276758) Establishment Name Address ID/FEI Business Operations Quality Care Products LLC 831276758 relabel(35356-197)