Label: CEPACOL ANTIBACTERIAL- cetylpyridinium chloride liquid
- NDC Code(s): 63824-790-80
- Packager: RB Health (US) LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 18, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
- Warnings
- Directions
- Other Information
- Inactive ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
-
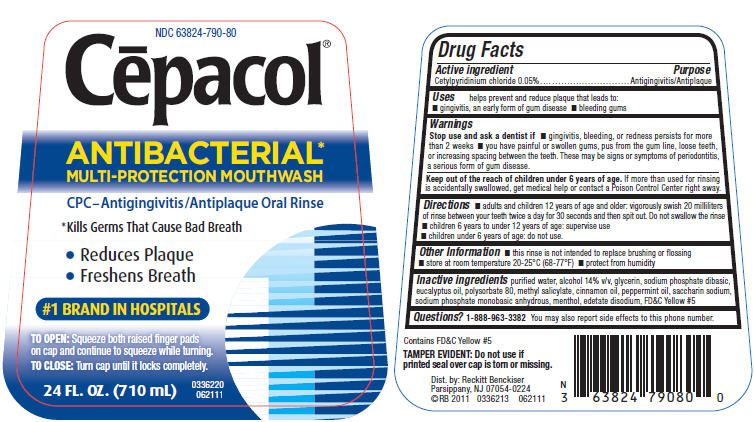
PRINCIPAL DISPLAY PANEL - 710 mL Bottle Label
NDC 63824-790-80
Cēpacol®
ANTIBACTERIAL*
MULTI-PROTECTION MOUTHWASHCPC–Antigingivitis/Antiplaque Oral Rinse
*Kills Germs That Cause Bad Breath
- Reduces Plaque
- Freshens Breath
#1 BRAND IN HOSPITALS
TO OPEN:Squeeze both raised finger pads
on cap and continue to squeeze while turning.TO CLOSE:Turn cap until it locks completely.
24 FL. OZ. (710 mL)
0336220
062111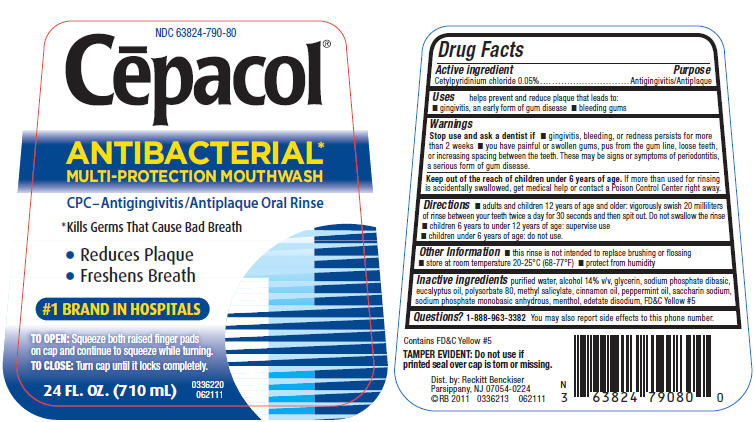
-
INGREDIENTS AND APPEARANCE
CEPACOL ANTIBACTERIAL
cetylpyridinium chloride liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63824-790 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CETYLPYRIDINIUM CHLORIDE (UNII: D9OM4SK49P) (CETYLPYRIDINIUM - UNII:CUB7JI0JV3) CETYLPYRIDINIUM CHLORIDE 0.05 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) GLYCERIN (UNII: PDC6A3C0OX) SODIUM PHOSPHATE, DIBASIC, UNSPECIFIED FORM (UNII: GR686LBA74) EUCALYPTUS OIL (UNII: 2R04ONI662) POLYSORBATE 80 (UNII: 6OZP39ZG8H) METHYL SALICYLATE (UNII: LAV5U5022Y) CINNAMON OIL (UNII: E5GY4I6YCZ) PEPPERMINT OIL (UNII: AV092KU4JH) SACCHARIN SODIUM (UNII: SB8ZUX40TY) SODIUM PHOSPHATE, MONOBASIC, ANHYDROUS (UNII: KH7I04HPUU) MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) EDETATE DISODIUM (UNII: 7FLD91C86K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63824-790-80 1 in 1 CARTON 06/22/2011 1 710 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 06/22/2011 Labeler - RB Health (US) LLC (081049410)