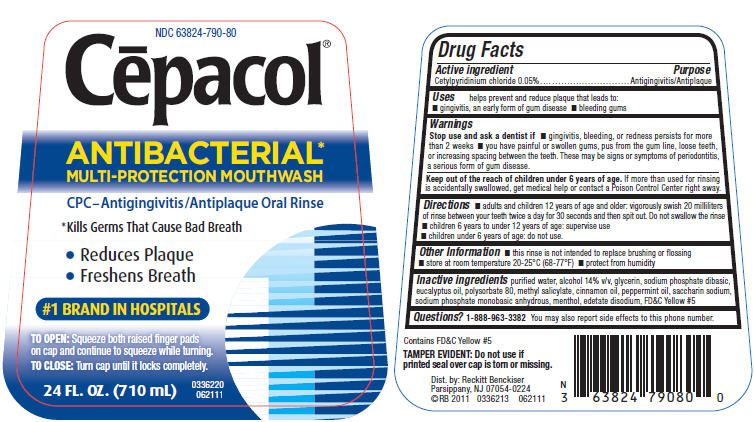
Label: CEPACOL ANTIBACTERIAL- cetylpyridinium chloride liquid
- NDC Code(s): 63824-790-80
- Packager: RB Health (US) LLC
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated June 22, 2022
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
- Warnings
- Directions
- Other Information
- Inactive ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - 710 mL Bottle Label
NDC 63824-790-80
Cēpacol®
ANTIBACTERIAL*
MULTI-PROTECTION MOUTHWASHCPC–Antigingivitis/Antiplaque Oral Rinse
*Kills Germs That Cause Bad Breath
- Reduces Plaque
- Freshens Breath
#1 BRAND IN HOSPITALS
TO OPEN: Squeeze both raised finger pads
on cap and continue to squeeze while turning.TO CLOSE: Turn cap until it locks completely.
24 FL. OZ. (710 mL)
0336220
062111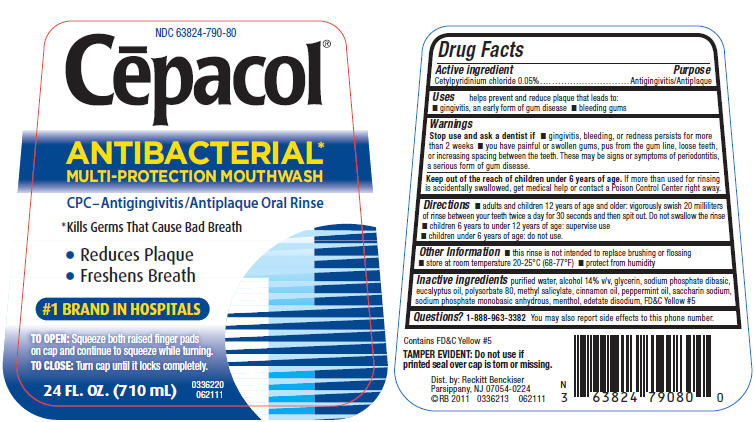
-
INGREDIENTS AND APPEARANCE
CEPACOL ANTIBACTERIAL
cetylpyridinium chloride liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63824-790 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Cetylpyridinium chloride (UNII: D9OM4SK49P) (Cetylpyridinium - UNII:CUB7JI0JV3) Cetylpyridinium chloride 0.05 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) GLYCERIN (UNII: PDC6A3C0OX) SODIUM PHOSPHATE, DIBASIC, UNSPECIFIED FORM (UNII: GR686LBA74) EUCALYPTUS OIL (UNII: 2R04ONI662) POLYSORBATE 80 (UNII: 6OZP39ZG8H) METHYL SALICYLATE (UNII: LAV5U5022Y) CINNAMON OIL (UNII: E5GY4I6YCZ) PEPPERMINT OIL (UNII: AV092KU4JH) SACCHARIN SODIUM (UNII: SB8ZUX40TY) SODIUM PHOSPHATE, MONOBASIC, ANHYDROUS (UNII: KH7I04HPUU) MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) EDETATE DISODIUM (UNII: 7FLD91C86K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63824-790-80 1 in 1 CARTON 06/22/2011 1 710 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part356 06/22/2011 Labeler - RB Health (US) LLC (081049410)