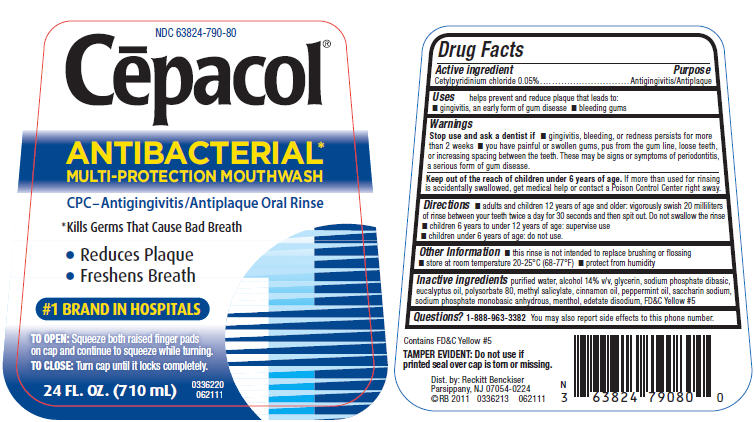
Uses
helps prevent and reduce plaque that leads to:
- gingivitis, an early form of gum disease
- bleeding gums
Warnings
Directions
- adults and children 12 years of age and older: vigorously swish 20 milliliters of rinse between your teeth twice a day for 30 seconds and then spit out. Do not swallow the rinse
- children 6 years to under 12 years of age: supervise use
- children under 6 years of age: do not use.
Other Information
- this rinse is not intended to replace brushing or flossing
- store at room temperature 20-25°C (68-77°F)
- protect from humidity
Inactive ingredients
purified water, alcohol 14% v/v, glycerin, sodium phosphate dibasic, eucalyptus oil, polysorbate 80, methyl salicylate, cinnamon oil, peppermint oil, saccharin sodium, sodium phosphate monobasic anhydrous, menthol, edetate disodium, FD&C Yellow #5
PRINCIPAL DISPLAY PANEL - 710 mL Bottle Label
NDC 63824-790-80
Cēpacol®
ANTIBACTERIAL*
MULTI-PROTECTION MOUTHWASH
CPC–Antigingivitis/Antiplaque Oral Rinse
*Kills Germs That Cause Bad Breath
- Reduces Plaque
- Freshens Breath
#1 BRAND IN HOSPITALS
TO OPEN: Squeeze both raised finger pads
on cap and continue to squeeze while turning.
TO CLOSE: Turn cap until it locks completely.
24 FL. OZ. (710 mL)
0336220
062111