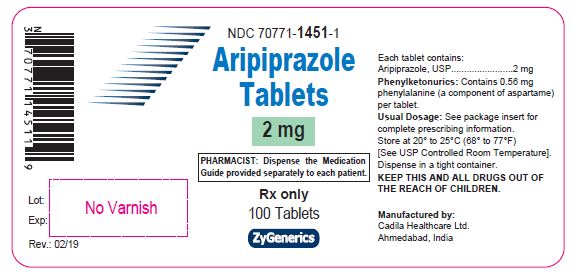
Label: ARIPIPRAZOLE tablet
-
NDC Code(s):
70771-1451-1,
70771-1451-3,
70771-1451-4,
70771-1451-5, view more70771-1452-1, 70771-1452-3, 70771-1453-0, 70771-1453-3, 70771-1453-6, 70771-1453-7, 70771-1453-9, 70771-1454-0, 70771-1454-3, 70771-1454-6, 70771-1454-7, 70771-1454-9, 70771-1455-0, 70771-1455-3, 70771-1455-6, 70771-1455-7, 70771-1455-9, 70771-1456-0, 70771-1456-3, 70771-1456-6, 70771-1456-7, 70771-1456-9
- Packager: Zydus Lifesciences Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated September 20, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- SPL MEDGUIDE
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ARIPIPRAZOLE
aripiprazole tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:70771-1452 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARIPIPRAZOLE (UNII: 82VFR53I78) (ARIPIPRAZOLE - UNII:82VFR53I78) ARIPIPRAZOLE 5 mg Inactive Ingredients Ingredient Name Strength ASPARTAME (UNII: Z0H242BBR1) CALCIUM STEARATE (UNII: 776XM7047L) CROSPOVIDONE (UNII: 2S7830E561) PEPPERMINT (UNII: V95R5KMY2B) MANNITOL (UNII: 3OWL53L36A) Product Characteristics Color WHITE (WHITE TO OFF-WHITE) Score no score Shape FREEFORM (BARREL) Size 8mm Flavor PEPPERMINT (FLAVOR FIRMENICH POWDER PEPPERMINT) Imprint Code ZE;13 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70771-1452-3 30 in 1 BOTTLE; Type 0: Not a Combination Product 06/04/2019 2 NDC:70771-1452-1 100 in 1 BOTTLE; Type 0: Not a Combination Product 06/04/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA090472 06/04/2019 ARIPIPRAZOLE
aripiprazole tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:70771-1453 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARIPIPRAZOLE (UNII: 82VFR53I78) (ARIPIPRAZOLE - UNII:82VFR53I78) ARIPIPRAZOLE 10 mg Inactive Ingredients Ingredient Name Strength ASPARTAME (UNII: Z0H242BBR1) CALCIUM STEARATE (UNII: 776XM7047L) CROSPOVIDONE (UNII: 2S7830E561) PEPPERMINT (UNII: V95R5KMY2B) MANNITOL (UNII: 3OWL53L36A) Product Characteristics Color WHITE (WHITE TO OFF-WHITE) Score no score Shape CAPSULE (CAPSULE) Size 8mm Flavor PEPPERMINT (FLAVOR FIRMENICH POWDER PEPPERMINT) Imprint Code ZE;4 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70771-1453-3 30 in 1 BOTTLE; Type 0: Not a Combination Product 06/04/2019 2 NDC:70771-1453-6 60 in 1 BOTTLE; Type 0: Not a Combination Product 06/04/2019 3 NDC:70771-1453-9 90 in 1 BOTTLE; Type 0: Not a Combination Product 06/04/2019 4 NDC:70771-1453-7 120 in 1 BOTTLE; Type 0: Not a Combination Product 06/04/2019 5 NDC:70771-1453-0 1000 in 1 BOTTLE; Type 0: Not a Combination Product 06/04/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA090472 06/04/2019 ARIPIPRAZOLE
aripiprazole tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:70771-1454 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARIPIPRAZOLE (UNII: 82VFR53I78) (ARIPIPRAZOLE - UNII:82VFR53I78) ARIPIPRAZOLE 15 mg Inactive Ingredients Ingredient Name Strength ASPARTAME (UNII: Z0H242BBR1) CALCIUM STEARATE (UNII: 776XM7047L) CROSPOVIDONE (UNII: 2S7830E561) PEPPERMINT (UNII: V95R5KMY2B) MANNITOL (UNII: 3OWL53L36A) Product Characteristics Color WHITE (WHITE TO OFF-WHITE) Score no score Shape ROUND (ROUND) Size 8mm Flavor PEPPERMINT (FLAVOR FIRMENICH POWDER PEPPERMINT) Imprint Code ZE;14 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70771-1454-3 30 in 1 BOTTLE; Type 0: Not a Combination Product 06/04/2019 2 NDC:70771-1454-6 60 in 1 BOTTLE; Type 0: Not a Combination Product 06/04/2019 3 NDC:70771-1454-9 90 in 1 BOTTLE; Type 0: Not a Combination Product 06/04/2019 4 NDC:70771-1454-7 120 in 1 BOTTLE; Type 0: Not a Combination Product 06/04/2019 5 NDC:70771-1454-0 1000 in 1 BOTTLE; Type 0: Not a Combination Product 06/04/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA090472 06/04/2019 ARIPIPRAZOLE
aripiprazole tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:70771-1455 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARIPIPRAZOLE (UNII: 82VFR53I78) (ARIPIPRAZOLE - UNII:82VFR53I78) ARIPIPRAZOLE 20 mg Inactive Ingredients Ingredient Name Strength ASPARTAME (UNII: Z0H242BBR1) CALCIUM STEARATE (UNII: 776XM7047L) CROSPOVIDONE (UNII: 2S7830E561) PEPPERMINT (UNII: V95R5KMY2B) MANNITOL (UNII: 3OWL53L36A) Product Characteristics Color WHITE (WHITE TO OFF-WHITE) Score no score Shape FREEFORM (BARREL) Size 10mm Flavor PEPPERMINT (FLAVOR FIRMENICH POWDER PEPPERMINT) Imprint Code ZE;15 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70771-1455-3 30 in 1 BOTTLE; Type 0: Not a Combination Product 06/04/2019 2 NDC:70771-1455-6 60 in 1 BOTTLE; Type 0: Not a Combination Product 06/04/2019 3 NDC:70771-1455-9 90 in 1 BOTTLE; Type 0: Not a Combination Product 06/04/2019 4 NDC:70771-1455-7 120 in 1 BOTTLE; Type 0: Not a Combination Product 06/04/2019 5 NDC:70771-1455-0 1000 in 1 BOTTLE; Type 0: Not a Combination Product 06/04/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA090472 06/04/2019 ARIPIPRAZOLE
aripiprazole tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:70771-1456 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARIPIPRAZOLE (UNII: 82VFR53I78) (ARIPIPRAZOLE - UNII:82VFR53I78) ARIPIPRAZOLE 30 mg Inactive Ingredients Ingredient Name Strength ASPARTAME (UNII: Z0H242BBR1) CALCIUM STEARATE (UNII: 776XM7047L) CROSPOVIDONE (UNII: 2S7830E561) PEPPERMINT (UNII: V95R5KMY2B) MANNITOL (UNII: 3OWL53L36A) Product Characteristics Color WHITE (WHITE TO OFF-WHITE) Score no score Shape ROUND (ROUND) Size 10mm Flavor PEPPERMINT (FLAVOR FIRMENICH POWDER PEPPERMINT) Imprint Code ZE;16 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70771-1456-3 30 in 1 BOTTLE; Type 0: Not a Combination Product 06/04/2019 2 NDC:70771-1456-6 60 in 1 BOTTLE; Type 0: Not a Combination Product 06/04/2019 3 NDC:70771-1456-9 90 in 1 BOTTLE; Type 0: Not a Combination Product 06/04/2019 4 NDC:70771-1456-7 120 in 1 BOTTLE; Type 0: Not a Combination Product 06/04/2019 5 NDC:70771-1456-0 1000 in 1 BOTTLE; Type 0: Not a Combination Product 06/04/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA090472 06/04/2019 ARIPIPRAZOLE
aripiprazole tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:70771-1451 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARIPIPRAZOLE (UNII: 82VFR53I78) (ARIPIPRAZOLE - UNII:82VFR53I78) ARIPIPRAZOLE 2 mg Inactive Ingredients Ingredient Name Strength ASPARTAME (UNII: Z0H242BBR1) CALCIUM STEARATE (UNII: 776XM7047L) CROSPOVIDONE (UNII: 2S7830E561) MANNITOL (UNII: 3OWL53L36A) Product Characteristics Color WHITE (WHITE TO OFF-WHITE) Score no score Shape CAPSULE (CAPSULE) Size 6mm Flavor Imprint Code L1 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70771-1451-3 30 in 1 BOTTLE; Type 0: Not a Combination Product 06/04/2019 2 NDC:70771-1451-1 100 in 1 BOTTLE; Type 0: Not a Combination Product 06/04/2019 3 NDC:70771-1451-5 500 in 1 BOTTLE; Type 0: Not a Combination Product 06/04/2019 4 NDC:70771-1451-4 10 in 1 CARTON 06/04/2019 4 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA090472 06/04/2019 Labeler - Zydus Lifesciences Limited (918596198) Registrant - Zydus Lifesciences Limited (918596198) Establishment Name Address ID/FEI Business Operations Zydus Lifesciences Limited 918596198 ANALYSIS(70771-1451, 70771-1452, 70771-1453, 70771-1454, 70771-1455, 70771-1456) , MANUFACTURE(70771-1451, 70771-1452, 70771-1453, 70771-1454, 70771-1455, 70771-1456)