PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
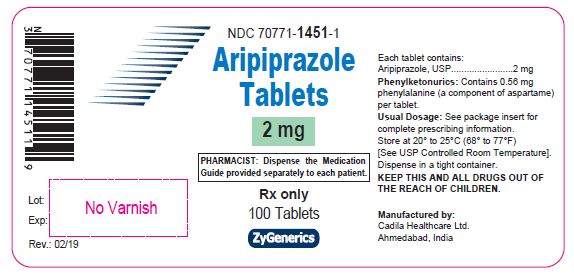
Aripiprazole Tablets, 2 mg
100 tablets
Rx only
Aripiprazole Tablets, 5 mg
100 tablets
Rx only

Aripiprazole Tablets, 10 mg
90 tablets
Rx only

Aripiprazole Tablets, 15 mg
90 tablets
Rx only

Aripiprazole Tablets, 20 mg
90 tablets
Rx only

Aripiprazole Tablets, 30 mg
90 tablets
Rx only
