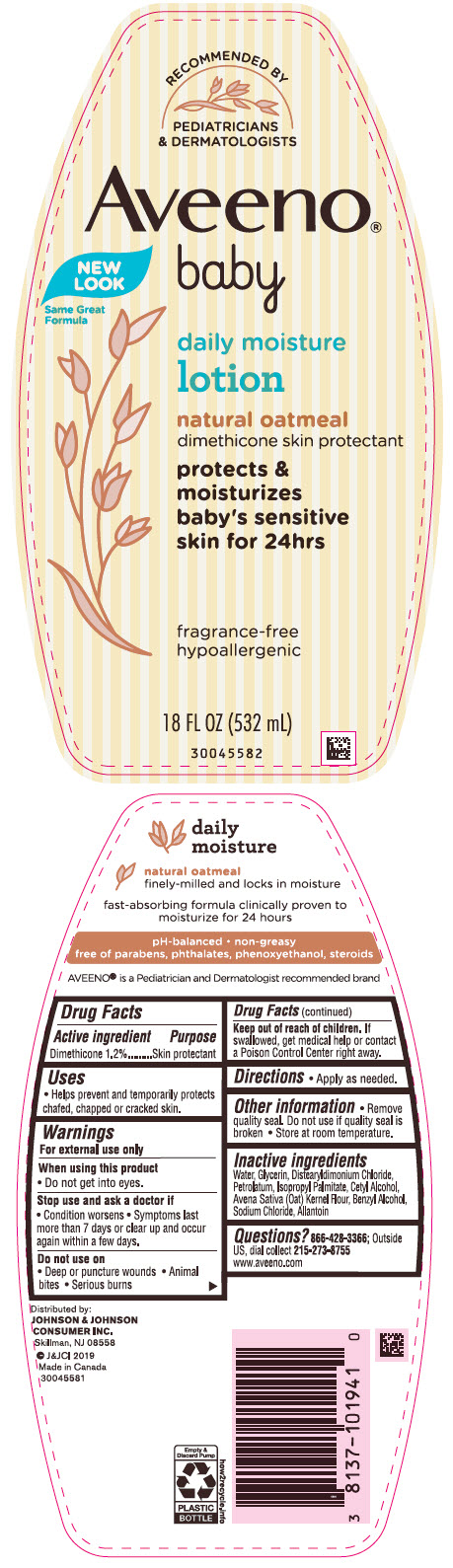
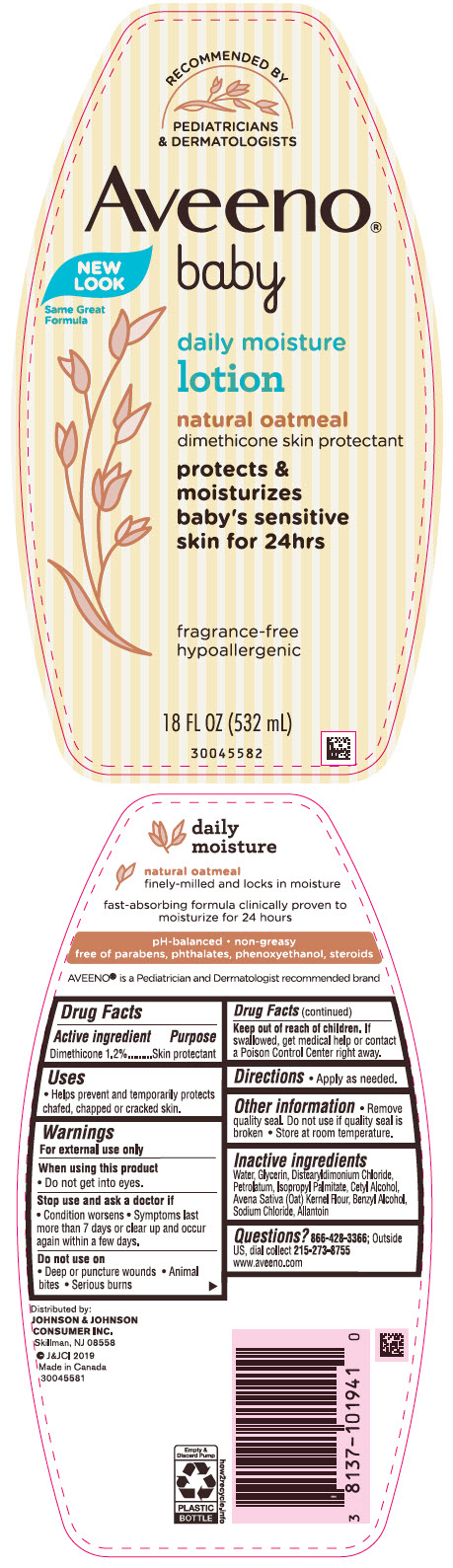
Label: AVEENO BABY DAILY MOISTURE- dimethicone lotion
-
NDC Code(s):
69968-0441-1,
69968-0441-2,
69968-0441-3,
69968-0441-4, view more69968-0441-8, 69968-0441-9
- Packager: Johnson & Johnson Consumer Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 21, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
- Warnings
- Directions
- Other information
- Inactive ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 532 mL Bottle Label
-
INGREDIENTS AND APPEARANCE
AVEENO BABY DAILY MOISTURE
dimethicone lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0441 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIMETHICONE (UNII: 92RU3N3Y1O) (DIMETHICONE - UNII:92RU3N3Y1O) DIMETHICONE 12 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) DISTEARYLDIMONIUM CHLORIDE (UNII: OM9573ZX3X) PETROLATUM (UNII: 4T6H12BN9U) ISOPROPYL PALMITATE (UNII: 8CRQ2TH63M) CETYL ALCOHOL (UNII: 936JST6JCN) OAT (UNII: Z6J799EAJK) BENZYL ALCOHOL (UNII: LKG8494WBH) SODIUM CHLORIDE (UNII: 451W47IQ8X) ALLANTOIN (UNII: 344S277G0Z) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0441-8 227 mL in 1 TUBE; Type 0: Not a Combination Product 08/01/2001 06/30/2020 2 NDC:69968-0441-1 532 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 08/01/2001 3 NDC:69968-0441-2 354 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 08/01/2001 4 NDC:69968-0441-3 29 mL in 1 BOTTLE; Type 0: Not a Combination Product 08/01/2001 07/02/2024 5 NDC:69968-0441-4 24 in 1 CARTON 08/01/2001 5 29 mL in 1 BOTTLE; Type 0: Not a Combination Product 6 NDC:69968-0441-9 2 in 1 PACKAGE 08/01/2001 6 532 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 08/01/2001 Labeler - Johnson & Johnson Consumer Inc. (118772437)