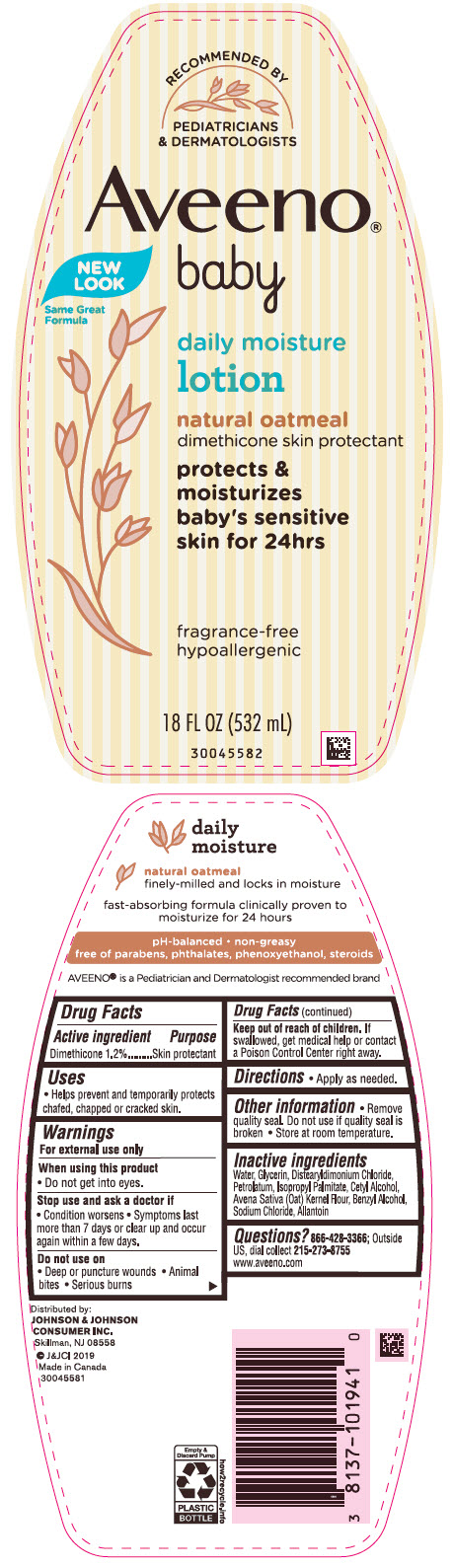
Warnings
For external use only
Other information
- Remove quality seal. Do not use if quality seal is broken.
- Store at room temperature.
Inactive ingredients
Water, Glycerin, Distearyldimonium Chloride, Petrolatum, Isopropyl Palmitate, Cetyl Alcohol, Avena Sativa (Oat) Kernel Flour, Benzyl Alcohol, Sodium Chloride, Allantoin
PRINCIPAL DISPLAY PANEL - 532 mL Bottle Label
Recommended By
PEDIATRICIANS
& DERMATOLOGISTS
Aveeno ®
baby
NEW
LOOK
Same Great
Formula
daily moisture
lotion
natural oatmeal
dimethicone skin protectant
protects &
moisturizes
baby's sensitive
skin for 24hrs
fragrance-free
hypoallergenic
18 FL OZ (532 mL)