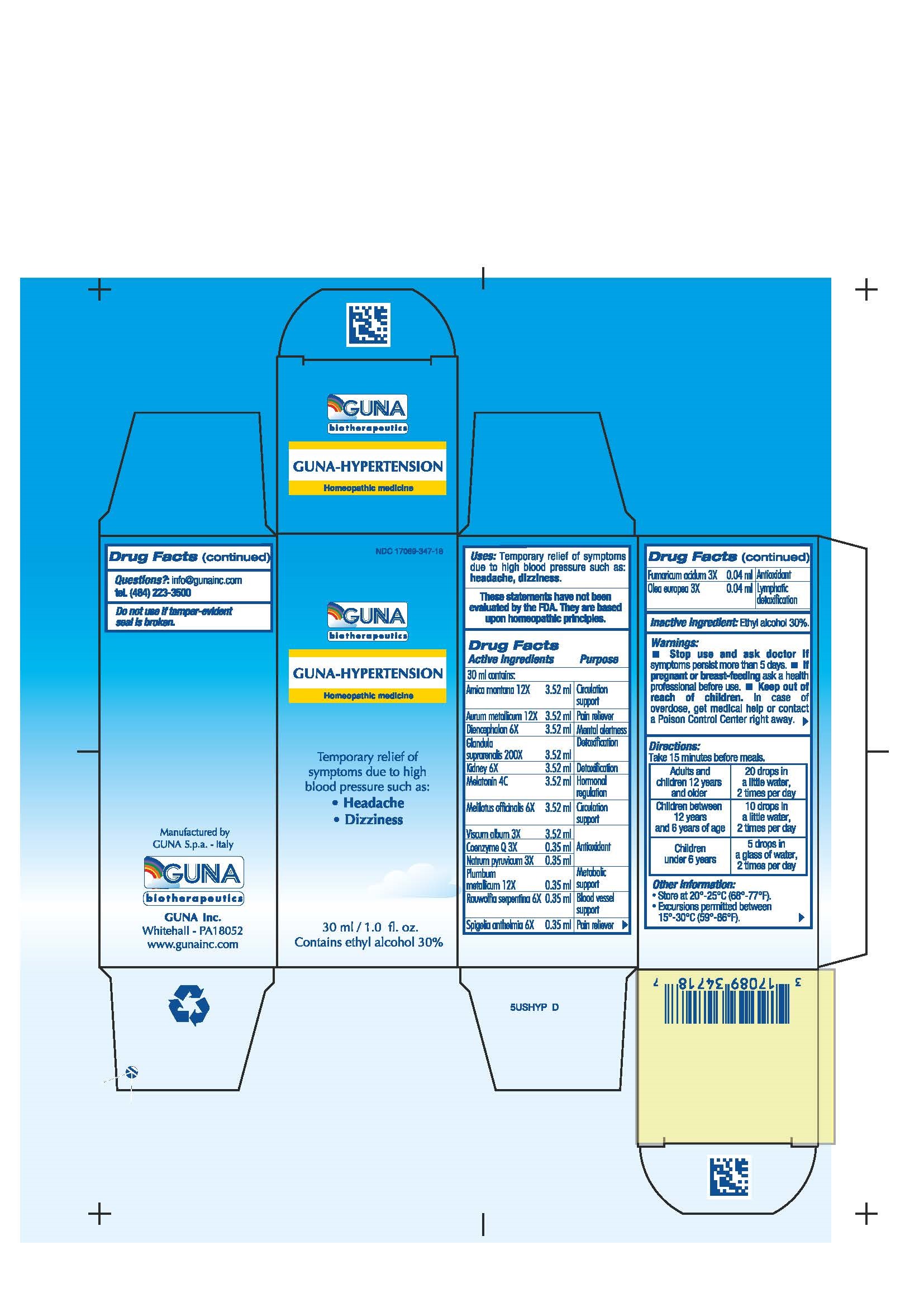
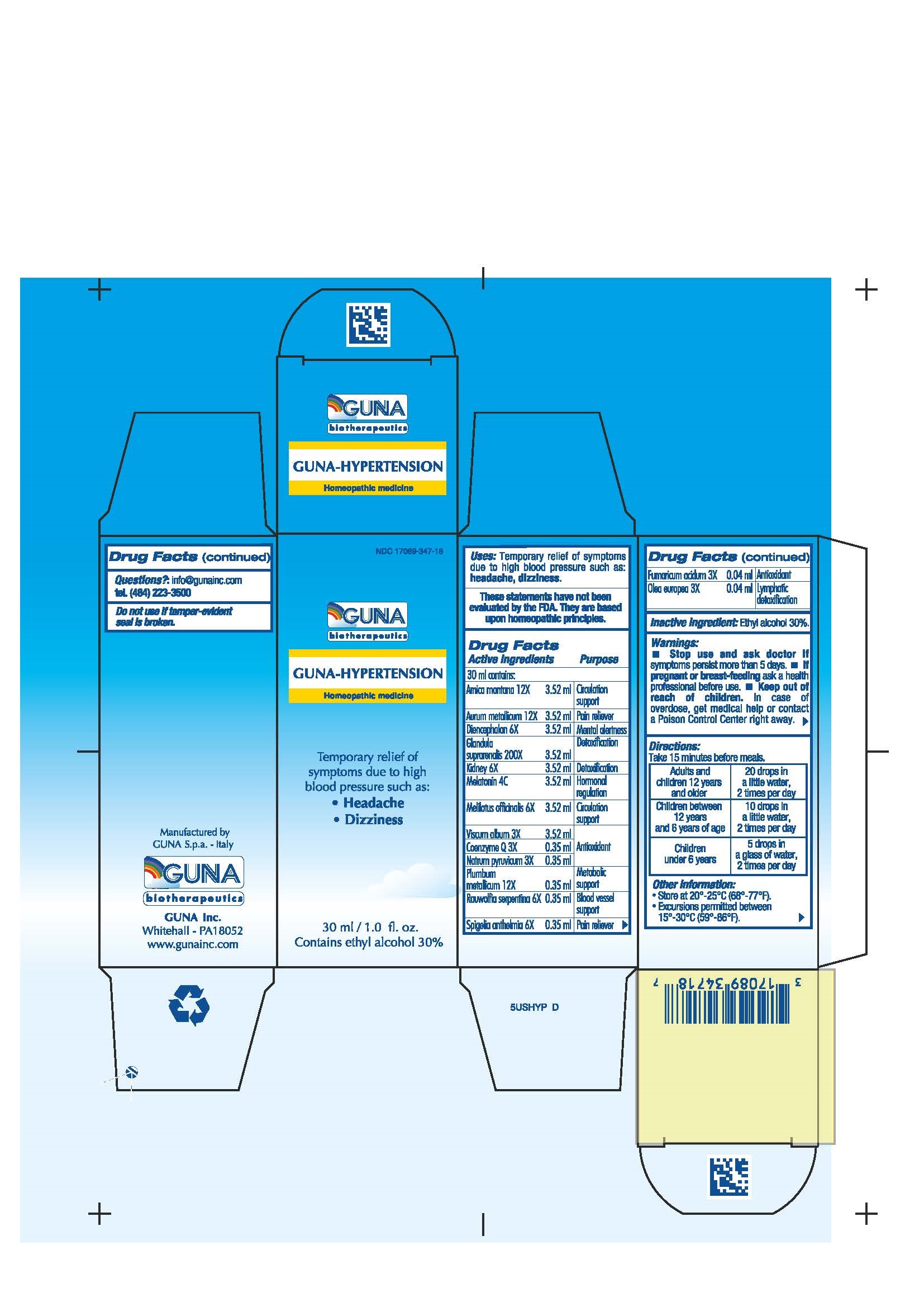
Label: GUNA-HYPERTENSION- arnica montana - fumaric acid - gold - lead - melatonin - melilotus - olea europaea flower - pork kidney - rauwolfia serpentina - sodium pyruvate - spigelia - sus scrofa adrenal gland - sus scrofa diencephalon - ubidecarenone - viscum album fruit - solution/ drops
- NDC Code(s): 17089-347-18
- Packager: Guna spa
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated December 21, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
ACTIVE INGREDIENT
Arnica montana 12X Circulation support
Aurum metallicum 12X Pain reliever
Coenzyme Q 3X ANTIOXIDANT
Diencephalon 6X MENTAL ALERTNESS
Fumaricum acidum 3X ANTIOXIDANT
Glandula suprarenalis 200X DETOXIFICATION
Kidney 6X DETOXIFICATION
Melatonin 4C HORMONAL REGULATION
Melilotus officinalis 6X Circulation support
Natrum pyruvicum 3X ANTIOXIDANT
Olea europea 3X LYMPHATIC DETOXIFICATION
Plumbum metallicum 12X Metabolic support
Rauwolfia serpentina 6X BLOOD VESSEL SUPPORT
Spigelia anthelmia 6X Pain reliever
Viscum album 3X ANTIOXIDANT - PURPOSE
- WARNINGS
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- QUESTIONS
- INDICATIONS & USAGE
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GUNA-HYPERTENSION
arnica montana - fumaric acid - gold - lead - melatonin - melilotus - olea europaea flower - pork kidney - rauwolfia serpentina - sodium pyruvate - spigelia - sus scrofa adrenal gland - sus scrofa diencephalon - ubidecarenone - viscum album fruit - solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:17089-347 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARNICA MONTANA (UNII: O80TY208ZW) (ARNICA MONTANA - UNII:O80TY208ZW) ARNICA MONTANA 12 [hp_X] in 30 mL GOLD (UNII: 79Y1949PYO) (GOLD - UNII:79Y1949PYO) GOLD 12 [hp_X] in 30 mL UBIDECARENONE (UNII: EJ27X76M46) (UBIDECARENONE - UNII:EJ27X76M46) UBIDECARENONE 3 [hp_X] in 30 mL SUS SCROFA DIENCEPHALON (UNII: 23PJ4252VL) (SUS SCROFA DIENCEPHALON - UNII:23PJ4252VL) SUS SCROFA DIENCEPHALON 6 [hp_X] in 30 mL FUMARIC ACID (UNII: 88XHZ13131) (FUMARIC ACID - UNII:88XHZ13131) FUMARIC ACID 3 [hp_X] in 30 mL SUS SCROFA ADRENAL GLAND (UNII: 398IYQ16YV) (SUS SCROFA ADRENAL GLAND - UNII:398IYQ16YV) SUS SCROFA ADRENAL GLAND 200 [hp_X] in 30 mL PORK KIDNEY (UNII: X7BCI5P86H) (PORK KIDNEY - UNII:X7BCI5P86H) PORK KIDNEY 6 [hp_X] in 30 mL MELATONIN (UNII: JL5DK93RCL) (MELATONIN - UNII:JL5DK93RCL) MELATONIN 4 [hp_C] in 30 mL MELILOTUS (UNII: F22I9R6Q0X) (MELILOTUS - UNII:F22I9R6Q0X) MELILOTUS 6 [hp_X] in 30 mL SODIUM PYRUVATE (UNII: POD38AIF08) (PYRUVIC ACID - UNII:8558G7RUTR) SODIUM PYRUVATE 3 [hp_X] in 30 mL OLEA EUROPAEA FLOWER (UNII: 498M34P1VZ) (OLEA EUROPAEA FLOWER - UNII:498M34P1VZ) OLEA EUROPAEA FLOWER 3 [hp_X] in 30 mL LEAD (UNII: 2P299V784P) (LEAD - UNII:2P299V784P) LEAD 12 [hp_X] in 30 mL RAUWOLFIA SERPENTINA (UNII: H192N84N1G) (RAUWOLFIA SERPENTINA - UNII:H192N84N1G) RAUWOLFIA SERPENTINA 6 [hp_X] in 30 mL SPIGELIA (UNII: 467D26HS0B) (SPIGELIA - UNII:467D26HS0B) SPIGELIA 6 [hp_X] in 30 mL VISCUM ALBUM FRUIT (UNII: P83EQ521R3) (VISCUM ALBUM FRUIT - UNII:P83EQ521R3) VISCUM ALBUM FRUIT 3 [hp_X] in 30 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) 9 mL in 30 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:17089-347-18 1 in 1 BOX 12/21/2018 1 30 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 04/16/2008 Labeler - Guna spa (430538264) Establishment Name Address ID/FEI Business Operations Guna spa 338587646 manufacture(17089-347)