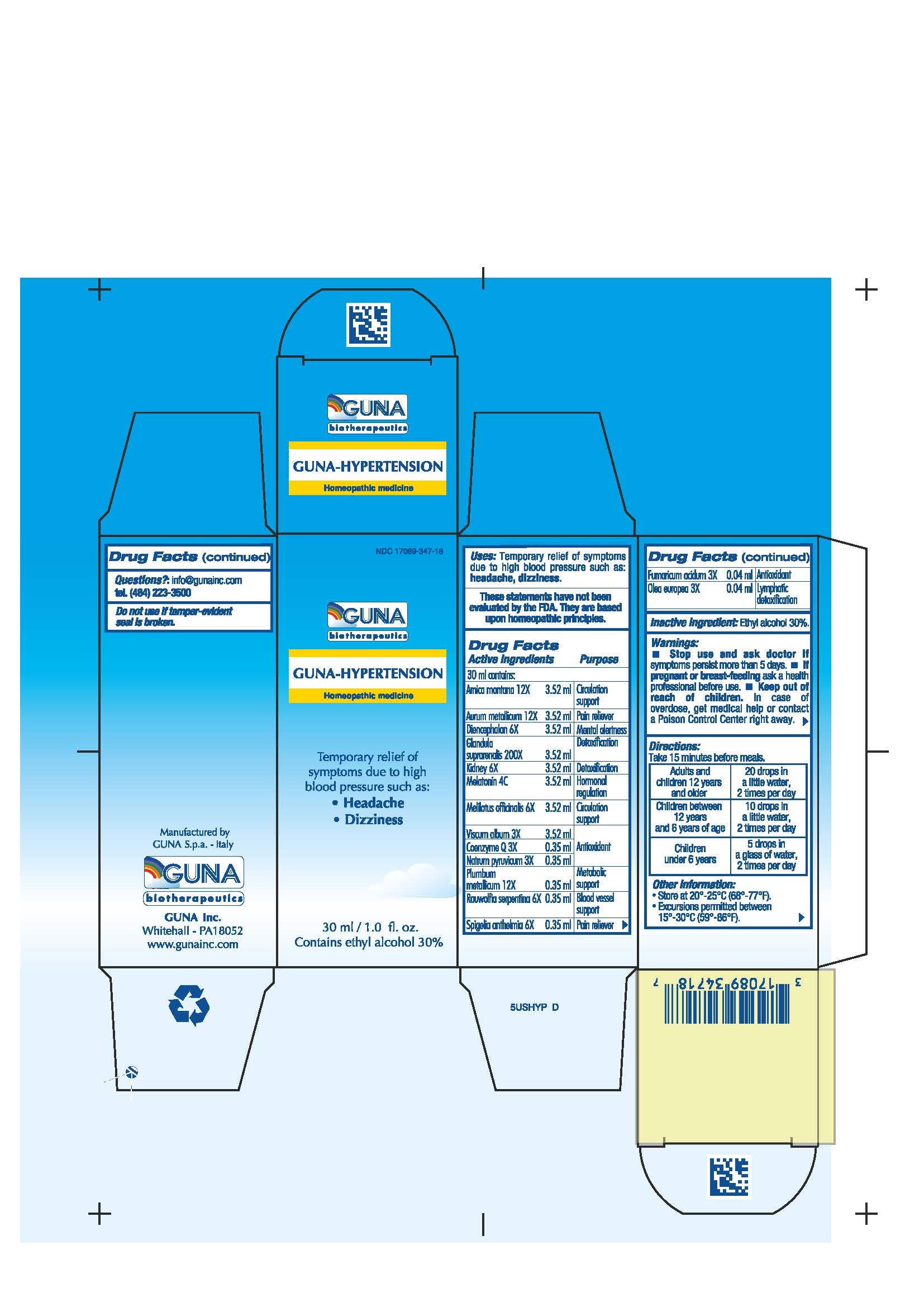
Arnica montana 12X Circulation support
Aurum metallicum 12X Pain reliever
Coenzyme Q 3X ANTIOXIDANT
Diencephalon 6X MENTAL ALERTNESS
Fumaricum acidum 3X ANTIOXIDANT
Glandula suprarenalis 200X DETOXIFICATION
Kidney 6X DETOXIFICATION
Melatonin 4C HORMONAL REGULATION
Melilotus officinalis 6X Circulation support
Natrum pyruvicum 3X ANTIOXIDANT
Olea europea 3X LYMPHATIC DETOXIFICATION
Plumbum metallicum 12X Metabolic support
Rauwolfia serpentina 6X BLOOD VESSEL SUPPORT
Spigelia anthelmia 6X Pain reliever
Viscum album 3X ANTIOXIDANT
- Stop use and ask doctor if symptoms persist more than 5 days.
- If pregnant or breast-feeding ask a health professional before use.
- Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
- Contains ethyl alcohol 30%