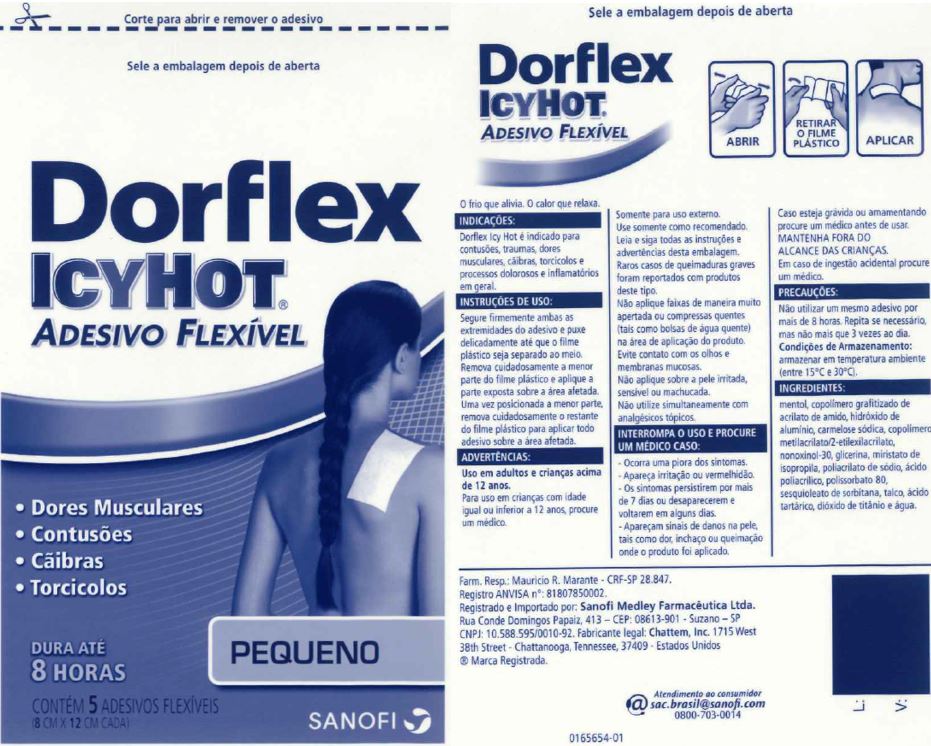
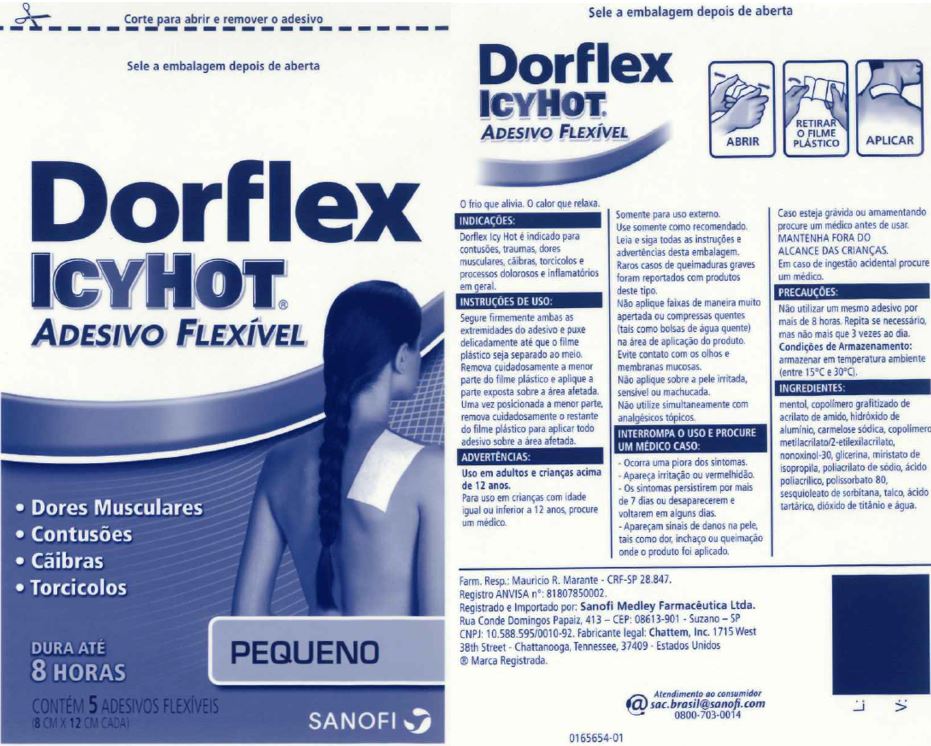
Label: DORFLEX ICY HOT FLEXIBLE, SMALL- menthol patch
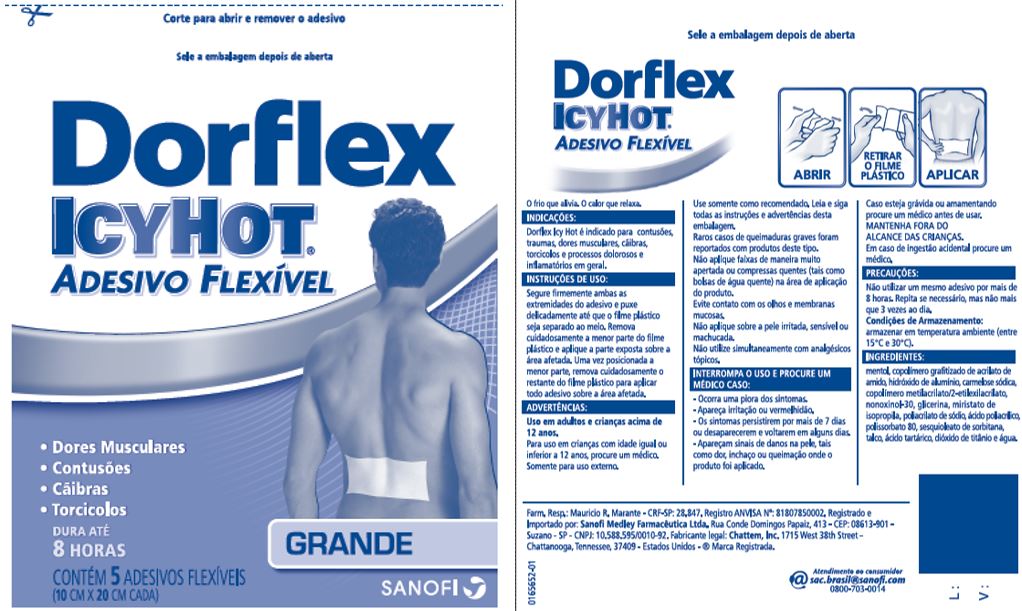
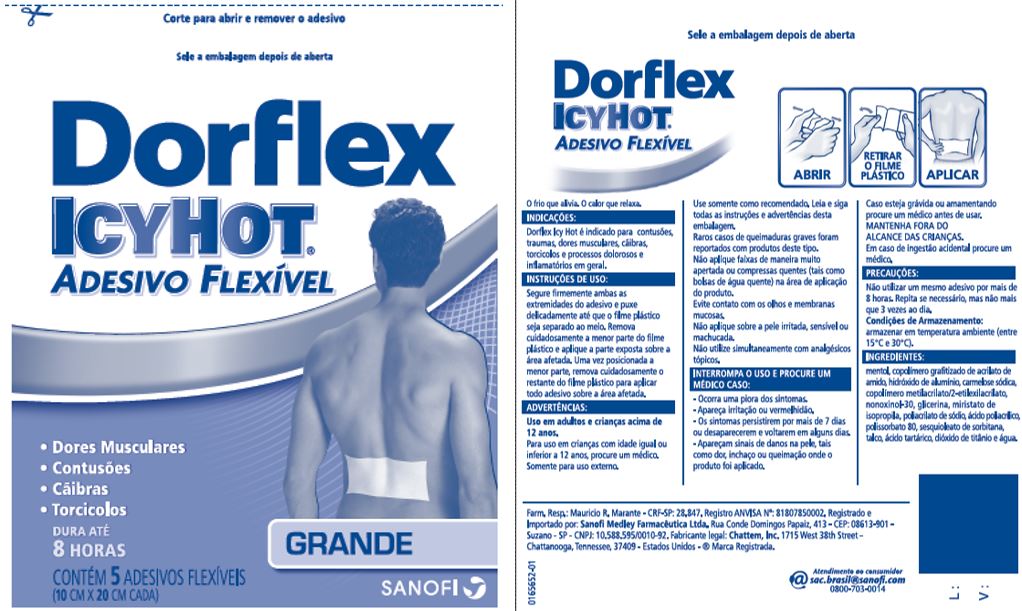
DORFLEX ICY HOT FLEXIBLE, LARGE- menthol patch
- NDC Code(s): 62168-0085-3, 62168-0086-6
- Packager: Lead Chemical Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 25, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Uses
-
Warnings
For external use only
For use in adults and children over 12 years.
When using this product
- •
- Use only as directed.
- •
- Do not use with a heating pad.
- •
- Avoid contact with eyes and mucous membranes.
- •
- Do not apply to wounds or damaged, broken or irritated skin.
-
Directions
- •
- Remove backing from patch by firmly grasping both ends and gently pulling until backing separates in middle
- •
- Carefully remove smaller portion of backing from patch and apply exposed portion of patch to affected area
- •
- Once exposed portion of patch is positioned, carefully remove remaining backing to completely apply patch to affected area
Children 12 years or younger, ask a doctor
- Precautions
- Inactive Ingredients
-
Other Information
Responsible pharmacist: Mauricio R. Marante - CRF-SP 28.847. ANVISA Registration No. 81807850002
Manufactured by: Lead Chemical Company, Ltd., 77-3 Himata, Toyama City, 930-0912. Toyama, Japan. Packaged by: Chattem, Inc., 1715 West 38th Street, Chattanooga, Tennessee, 37409. United States. Imported by: Sanofi Medley Farmaceutica Ltda. Rua Conde domingos Papaiz, 413 - Suzano - SP - CEP 08613- 901. CNPJ 10.588.595/0010-92.
- Principal Display Panel - Small
- Principal Display Panel - Large
-
INGREDIENTS AND APPEARANCE
DORFLEX ICY HOT FLEXIBLE, SMALL
menthol patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62168-0085 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 205.5 mg Inactive Ingredients Ingredient Name Strength ACRYLIC ACID (UNII: J94PBK7X8S) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED FORM (UNII: K679OBS311) 2-ETHYLHEXYL ACRYLATE (UNII: HR49R9S6XG) GLYCERIN (UNII: PDC6A3C0OX) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) METHYL ACRYLATE (UNII: WC487PR91H) NONOXYNOL-30 (UNII: JJX07DG188) SODIUM POLYACRYLATE (2500000 MW) (UNII: 05I15JNI2J) POLYACRYLIC ACID (250000 MW) (UNII: 9G2MAD7J6W) POLYSORBATE 80 (UNII: 6OZP39ZG8H) SORBITAN SESQUIOLEATE (UNII: 0W8RRI5W5A) STARCH, CORN (UNII: O8232NY3SJ) TALC (UNII: 7SEV7J4R1U) TARTARIC ACID (UNII: W4888I119H) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62168-0085-3 270 in 1 BOX 04/22/2021 10/31/2024 1 5 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 07/01/2013 10/31/2024 DORFLEX ICY HOT FLEXIBLE, LARGE
menthol patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62168-0086 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 428.5 mg Inactive Ingredients Ingredient Name Strength ACRYLIC ACID (UNII: J94PBK7X8S) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED FORM (UNII: K679OBS311) 2-ETHYLHEXYL ACRYLATE (UNII: HR49R9S6XG) GLYCERIN (UNII: PDC6A3C0OX) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) METHYL ACRYLATE (UNII: WC487PR91H) NONOXYNOL-30 (UNII: JJX07DG188) POLYACRYLIC ACID (250000 MW) (UNII: 9G2MAD7J6W) POLYSORBATE 80 (UNII: 6OZP39ZG8H) SODIUM POLYACRYLATE (2500000 MW) (UNII: 05I15JNI2J) SORBITAN SESQUIOLEATE (UNII: 0W8RRI5W5A) STARCH, CORN (UNII: O8232NY3SJ) TALC (UNII: 7SEV7J4R1U) TARTARIC ACID (UNII: W4888I119H) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62168-0086-6 100 in 1 BOX 09/20/2020 11/30/2024 1 5 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 07/01/2013 11/30/2024 Labeler - Lead Chemical Co., Ltd. (693727091)