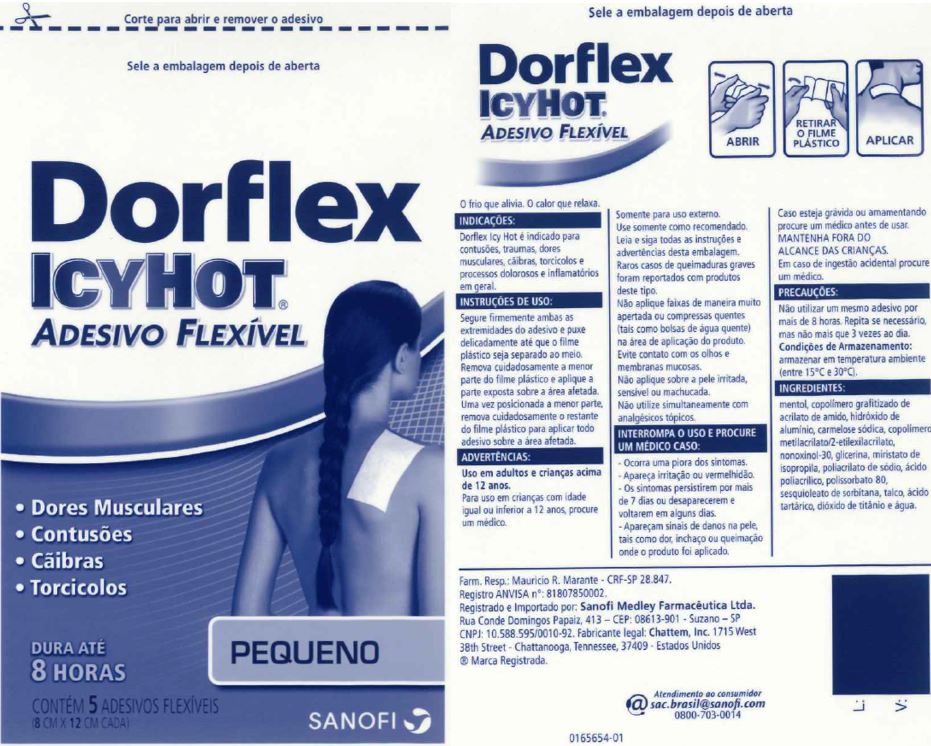
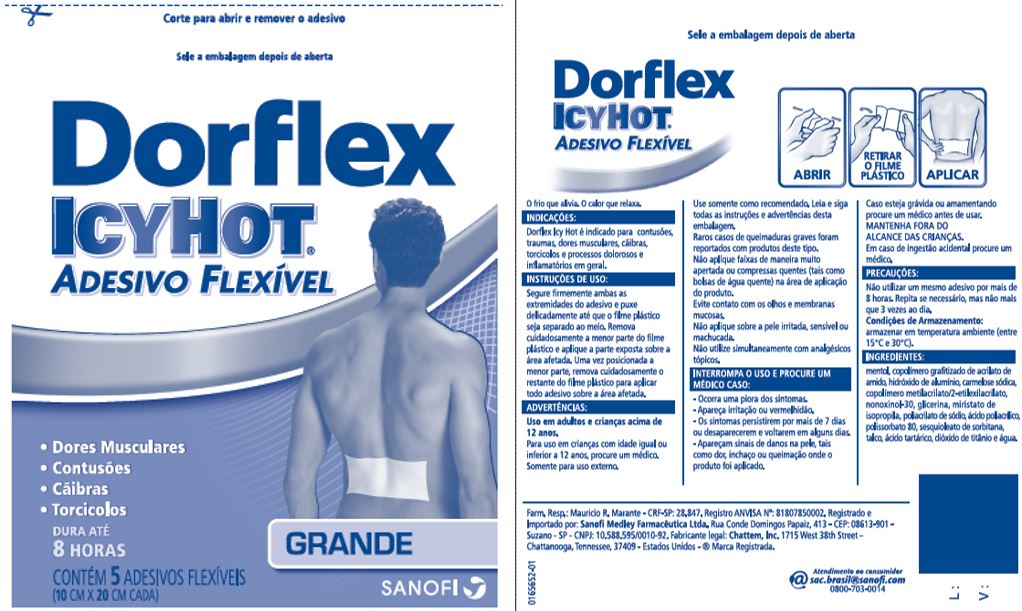
Uses
Dorflex Icy Hot is indicated for bruises, traumas, muscle pains, cramps, torticollis and painful and inflammatory processes at large.
Warnings
For external use only
For use in adults and children over 12 years.
When using this product
- •
- Use only as directed.
- •
- Do not use with a heating pad.
- •
- Avoid contact with eyes and mucous membranes.
- •
- Do not apply to wounds or damaged, broken or irritated skin.
Directions
- •
- Remove backing from patch by firmly grasping both ends and gently pulling until backing separates in middle
- •
- Carefully remove smaller portion of backing from patch and apply exposed portion of patch to affected area
- •
- Once exposed portion of patch is positioned, carefully remove remaining backing to completely apply patch to affected area
Children 12 years or younger, ask a doctor
Precautions
- •
- Do not wear the same patch for more than 8 hours.
- •
- Do not wear more than 3 patches daily.
- •
- Store in ambient temperature.
- •
- Use each patch only once.
Inactive Ingredients
acrylic acid, aluminum hydroxide, carmellose sodium, 2-ethylhexyl acrylate, glycerin, isopropyl myristate, methyl acrylate, nonoxynol-30, polyacrylate, polyacrylic acid, polysorbate 80, sorbitan sesquioleate, starch, talc, tartaric acid, titanium dioxide, water
Other Information
Responsible pharmacist: Mauricio R. Marante - CRF-SP 28.847. ANVISA Registration No. 81807850002
Manufactured by: Lead Chemical Company, Ltd., 77-3 Himata, Toyama City, 930-0912. Toyama, Japan. Packaged by: Chattem, Inc., 1715 West 38th Street, Chattanooga, Tennessee, 37409. United States. Imported by: Sanofi Medley Farmaceutica Ltda. Rua Conde domingos Papaiz, 413 - Suzano - SP - CEP 08613- 901. CNPJ 10.588.595/0010-92.