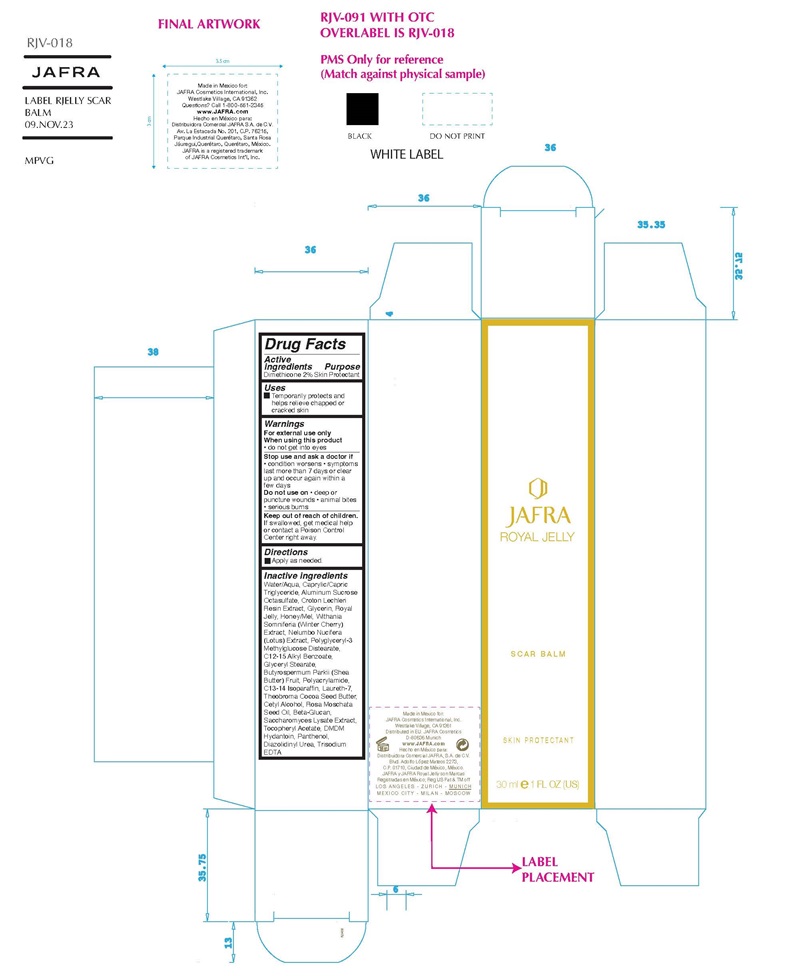
Label: ROYAL JELLY SCAR BALM- dimethicone cream
- NDC Code(s): 68828-004-01
- Packager: Jafra Cosmetics International Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 29, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
-
Warning
For external use only
When using this product● do not get into eyes
Stop use and ask a doctor if ● condition worsens ● symptoms last more than 7 days or clear up and occur again in a few days
Do not use on● deep or puncture wounds ● animal bites ● serious burns
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away. - Direction
- KEEP OUT OF REACH OF CHILDREN
-
Inactive ingredients
Water/Aqua, Caprylic/Capric Triglyceride, Aluminum Sucrose Octasulfate, Croton Lechleri Resin Extract, Glycerin, Royal Jelly, Honey/Mel, Withania Somniferia (Winter Cherry) Extract, Nelumbo Nucifera (Lotus) Extract, Polyglyceryl-3 Methylglucose Distearate, C12-15 Alkyl Benzoate, Glyceryl Stearate, Butyrospermum Parkii (Shea Butter) Fruit, Polyacrylamide, C13-14 Isoparaffin, Laureth-7, Theobroma Cocoa Seed Butter, Cetyl Alcohol, Rosa Moschata Seed Oil, Beta-Glucan, Saccharomyces Lysate Extract, Tocopheryl Acetate, DMDM Hydantoin, Panthenol, Diazolidinyl Urea, Trisodium EDTA
- Product label
-
INGREDIENTS AND APPEARANCE
ROYAL JELLY SCAR BALM
dimethicone creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68828-004 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIMETHICONE (UNII: 92RU3N3Y1O) (DIMETHICONE - UNII:92RU3N3Y1O) DIMETHICONE 2 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) SUCRALFATE (UNII: XX73205DH5) CROTON LECHLERI RESIN (UNII: GGG6W25C63) GLYCERIN (UNII: PDC6A3C0OX) ROYAL JELLY (UNII: L497I37F0C) HONEY (UNII: Y9H1V576FH) WITHANOLIDE D (UNII: XY366XV8JT) NELUMBO NUCIFERA WHOLE (UNII: 622D93A1R1) POLYGLYCERYL-3 METHYLGLUCOSE DISTEARATE (UNII: W19EIO0DBE) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) SHEA BUTTER (UNII: K49155WL9Y) POLYACRYLAMIDE (1500 MW) (UNII: 5D6TC4BRWV) C13-14 ISOPARAFFIN (UNII: E4F12ROE70) LAURETH-7 (UNII: Z95S6G8201) COCOA BUTTER (UNII: 512OYT1CRR) CETYL ALCOHOL (UNII: 936JST6JCN) ROSA MOSCHATA SEED OIL (UNII: T031ZE559T) YEAST .BETA.-D-GLUCAN (UNII: 44FQ49X6UN) SACCHAROMYCES LYSATE (UNII: R85W246Z1C) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) DMDM HYDANTOIN (UNII: BYR0546TOW) PANTHENOL (UNII: WV9CM0O67Z) DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) EDETATE TRISODIUM (UNII: 420IP921MB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68828-004-01 1 in 1 CARTON 06/15/2022 1 30 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 06/15/2022 Labeler - Jafra Cosmetics International Inc (041676479) Registrant - Jafra Cosmetics International Inc (041676479) Establishment Name Address ID/FEI Business Operations Distribuidora Comercial Jafra, S.A. de C.V. 951612777 manufacture(68828-004)