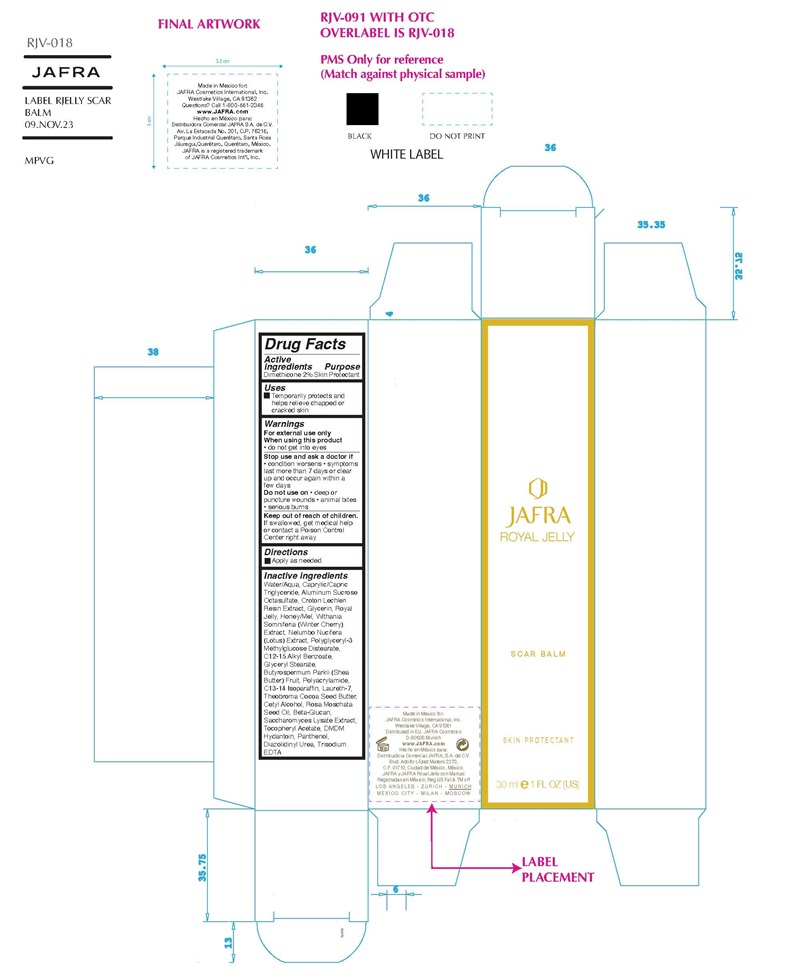
Warning
For external use only
When using this product● do not get into eyes
Stop use and ask a doctor if ● condition worsens ● symptoms last more than 7 days or clear up and occur again in a few days
Do not use on● deep or puncture wounds ● animal bites ● serious burns
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Inactive ingredients
Water/Aqua, Caprylic/Capric Triglyceride, Aluminum Sucrose Octasulfate, Croton Lechleri Resin Extract, Glycerin, Royal Jelly, Honey/Mel, Withania Somniferia (Winter Cherry) Extract, Nelumbo Nucifera (Lotus) Extract, Polyglyceryl-3 Methylglucose Distearate, C12-15 Alkyl Benzoate, Glyceryl Stearate, Butyrospermum Parkii (Shea Butter) Fruit, Polyacrylamide, C13-14 Isoparaffin, Laureth-7, Theobroma Cocoa Seed Butter, Cetyl Alcohol, Rosa Moschata Seed Oil, Beta-Glucan, Saccharomyces Lysate Extract, Tocopheryl Acetate, DMDM Hydantoin, Panthenol, Diazolidinyl Urea, Trisodium EDTA