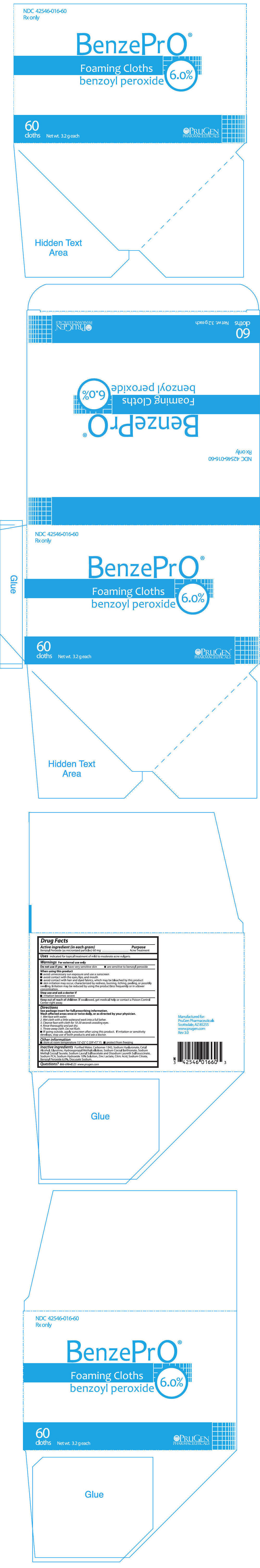
Label: BENZEPRO- benzoyl peroxide cloth
-
Contains inactivated NDC Code(s)
NDC Code(s): 42546-016-60 - Packager: PruGen, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated January 19, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
BenzePrO® Foaming Cloths are a topical preparation containing 6.0% benzoyl peroxide. Each gram of BenzePrO® Foaming Cloths wash contains 60 mg of Benzoyl Peroxide as micronized particles. Ingredients include Purified Water, Carbomer 1342, Sodium Hyaluronate, Cetyl Alcohol, Glycerine, Hydroxypropyl Methylcellulose, Sodium Cocoyl Isethionate, Sodium Methyl Cocoyl Taurate, Sodium Lauryl Sulfoacetate and Disodium Laureth Sulfosuccinate, Sodium PCA, Sodium Hydroxide 10% Solution, Zinc Lactate, Citric Acid, Sodium Citrate, Benzoyl Peroxide 6.0%, Docusate Sodium.
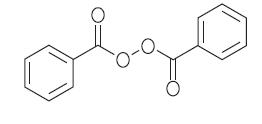
Benzoyl peroxide is an oxidizing agent that possesses antibacterial properties and is classified as a keratolytic. Benzoyl peroxide (C14H10O4) is represented by the following structure:
-
CLINICAL PHARMACOLOGY
The exact method of action of benzoyl peroxide in acne vulgaris is not known. Benzoyl peroxide is an antibacterial agent with demonstrated activity against Propionibacterium acnes. This action, combined with the mild keratolytic effect of benzoyl peroxide, is believed to be responsible for its usefulness in acne. Benzoyl peroxide is absorbed by the skin where it is metabolized to benzoic acid and excreted as benzoate in the urine.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
WARNINGS
FOR EXTERNAL USE ONLY. Not For Ophthalmic Use. Keep out of the reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
When using this product, skin irritation and dryness is more likely to occur if:
- you leave BenzePrO® Foaming Cloths on your skin longer than directed
- you use another topical acne medication at the same time
Do not use this product if you:
- have very sensitive skin
- are sensitive to benzoyl peroxide
When using this product:
- avoid unnecessary sun exposure and use a sunscreen
- avoid contact with the eyes, lips, mouth
- avoid contact with hair and dyed fabrics, which may be bleached by this product
- skin irritation may occur, characterized by redness, burning, itching, peeling, or possibly swelling. Irritation may be reduced by using the product less frequently or in lower concentration.
Stop use and ask a doctor:
- if irritation becomes severe.
-
PRECAUTIONS
Information for patients
This medication is to be used as directed by a physician and should not be used to treat any condition other than that for which it was prescribed. Avoid contact with eyes, eyelids, lips, and mucous membranes. If accidental contact occurs, rinse with water. If excessive redness or irritation develops, discontinue use and consult your physician.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Based upon all available evidence, benzoyl peroxide is not considered to be a carcinogen. However, data from a study using mice known to be highly susceptible to cancer suggest that benzoyl peroxide acts as a tumor promoter. The clinical significance of the findings is not known.
Pregnancy
Category C animal reproduction studies have not been conducted with benzoyl peroxide. It is also not known whether benzoyl peroxide can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Benzoyl peroxide should be used by a pregnant woman only if clearly needed.
- ADVERSE REACTIONS
-
OVERDOSAGE
If excessive scaling, erythema or edema occurs, the use of this preparation should be discontinued. To hasten resolution of the adverse effects, cool compresses may be used. After symptoms and signs subside, a reduced dosage schedule may be cautiously tried if the reaction is judged to be due to excessive use and not allergenicity.
-
DIRECTIONS
Avoid contact with hair, fabrics or carpeting as benzoyl peroxide will cause bleaching or discoloration.
Wash affected areas once or twice daily, or as directed by your physician.
- Wet face with water.
- Wet cloth with a little waterand work into a full lather.
- Cleanse face with cloth for 10-20 seconds avoiding eyes.
- Rinse thoroughly and pat dry.
- Throw away cloth. Do not flush.
- If going outside, apply sunscreen after using this product. If irritation or sensitivity develops, stop use of both products and ask a doctor.
- HOW SUPPLIED
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 60 Cloth Packet Carton
-
INGREDIENTS AND APPEARANCE
BENZEPRO
benzoyl peroxide clothProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:42546-016 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Benzoyl Peroxide (UNII: W9WZN9A0GM) (Benzoyl Peroxide - UNII:W9WZN9A0GM) Benzoyl Peroxide 60 mg Inactive Ingredients Ingredient Name Strength water (UNII: 059QF0KO0R) Carbomer Copolymer Type B (Allyl Pentaerythritol Crosslinked) (UNII: 809Y72KV36) Hyaluronate Sodium (UNII: YSE9PPT4TH) Cetyl Alcohol (UNII: 936JST6JCN) Glycerin (UNII: PDC6A3C0OX) HYPROMELLOSE 2910 (15 MPA.S) (UNII: 36SFW2JZ0W) Sodium Cocoyl Isethionate (UNII: 518XTE8493) Sodium Methyl Cocoyl Taurate (UNII: JVL98CG53G) Sodium Lauryl Sulfoacetate (UNII: D0Y70F2B9J) Disodium Laureth Sulfosuccinate (UNII: D6DH1DTN7E) Sodium Pyrrolidone Carboxylate (UNII: 469OTG57A2) Sodium Hydroxide (UNII: 55X04QC32I) Zinc Lactate (UNII: 2GXR25858Y) Citric Acid Monohydrate (UNII: 2968PHW8QP) Sodium Citrate, Unspecified Form (UNII: 1Q73Q2JULR) Docusate Sodium (UNII: F05Q2T2JA0) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:42546-016-60 60 in 1 CARTON 08/01/2015 1 1 in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED DRUG OTHER 08/01/2015 Labeler - PruGen, Inc. (929922750) Establishment Name Address ID/FEI Business Operations Span Packing Services 557434805 MANUFACTURE(42546-016)