Label: TVIA TOPICAL GEL- menthol, camphor gel
-
Contains inactivated NDC Code(s)
NDC Code(s): 72380-728-10 - Packager: Vivera Pharmaceuticals, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 5, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS AND PRECAUTIONS
- WARNINGS
- OTHER SAFETY INFORMATION
- ASK DOCTOR
- WHEN USING
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- OTHER SAFETY INFORMATION
-
INACTIVE INGREDIENT
INACTIVE INGREDIENTS: Ethyl Alcohol Denatured,
Deionized Water, Glycerin, Propylene Glycol, Cannabis
Sativa Seed Oil (Hemp Seed Oil), Cannabidiol (CBD), Arnica
Montana Flower Extract (Arnica Oil), Mentha Piperita
(Peppermint) Oil, Carbomer, Triethanolamine, Mentha Viridis
(Spearmint) Leaf Oil, Camellia Sinensis Leaf Extract, Aloe
Barbadensis Leaf Extract, Hamamelis Virginiana (Witch
Hazel) Extract, Melaleuca Alternifolia (Tea Tree) Leaf Oil, -
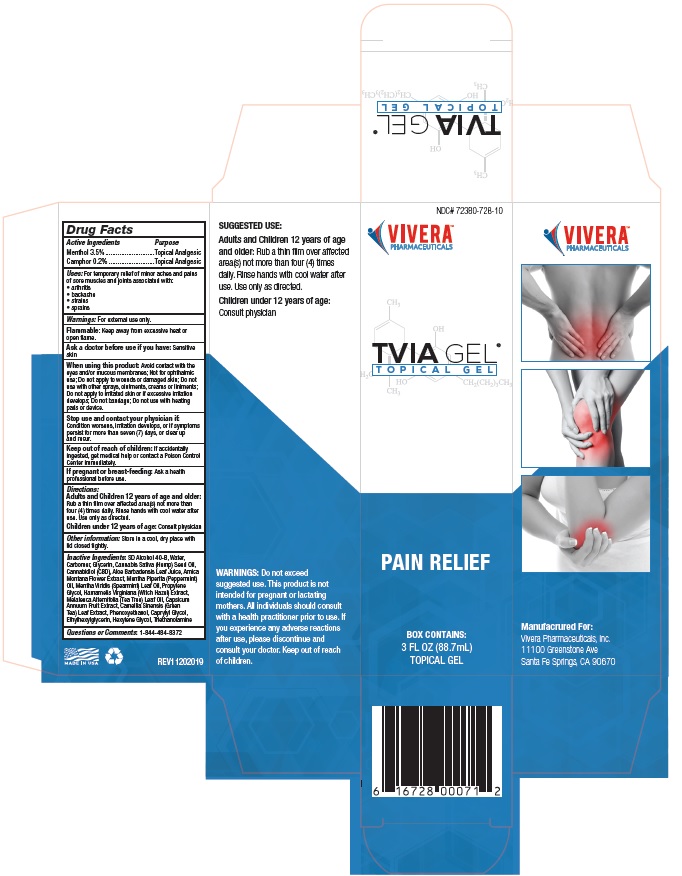
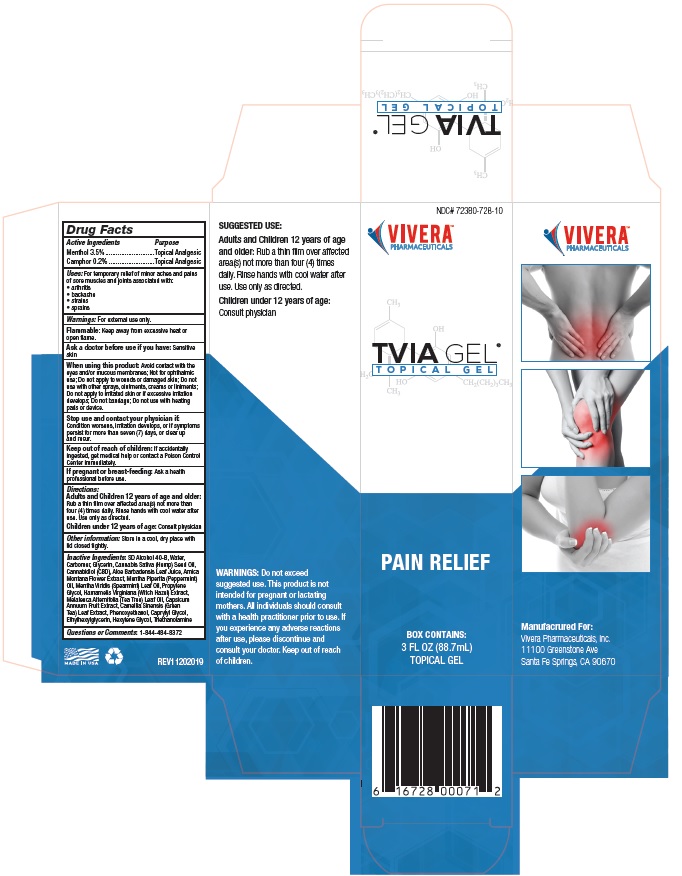
Principal Display Package
NDC# 72380-728-10
VIVERA®
PHARMACEUTICALSTVIA GEL®
T O P I C A L G E LPAIN RELIEF
BOX CONTAINS:
3 FL OZ (88.7mL)
TOPICAL GEL6 16728 00071 2
TVIA GEL®
T O P I C A L G E LSUGGESTED USE: Apply a
small amount topically to
affected area and rub in. Use
every 4 to 6 hours. Safe for
use on adults over 21 years
of age. Wash hands with cool
water after each use.WARNINGS: Do not exceed
suggested use. This product
is not intended for pregnant
or lactating mothers. All
individuals should consult with
a health practitioner prior
to use. If you experience any
adverse reactions after use,
please discontinue and consult
your doctor. Keep out of reach
of children. This product has
not been evaluated by the
Food and Drug Administration.VIVERA®
PHARMACEUTICALS*These statements have not been
evaluated by the Food and Drug
Administration. This product is not
intended to diagnose, treat, cure or
prevent any disease.VIV-71000-GBX REV20190115
VIVERA®
PHARMACEUTICALSManufacrured For:
Vivera Pharmaceuticals, Inc.
11100 Greenstone Ave
Santa Fe Springs, CA 90670MADE IN USA
Retail Box
Tube

Insert

TVIA Topical Gel by Vivera Pharmaceuticals, Inc.
res
-
INGREDIENTS AND APPEARANCE
TVIA TOPICAL GEL
menthol, camphor gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72380-728 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 3.5 g in 100 mL CAMPHOR (SYNTHETIC) (UNII: 5TJD82A1ET) (CAMPHOR (SYNTHETIC) - UNII:5TJD82A1ET) CAMPHOR (SYNTHETIC) 0.2 g in 100 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) CANNABIS SATIVA SEED OIL (UNII: 69VJ1LPN1S) CANNABIDIOL (UNII: 19GBJ60SN5) ARNICA MONTANA FLOWER (UNII: OZ0E5Y15PZ) PEPPERMINT OIL (UNII: AV092KU4JH) CARBOXYPOLYMETHYLENE (UNII: 0A5MM307FC) TROLAMINE (UNII: 9O3K93S3TK) SPEARMINT OIL (UNII: C3M81465G5) GREEN TEA LEAF (UNII: W2ZU1RY8B0) ALOE VERA LEAF (UNII: ZY81Z83H0X) HAMAMELIS VIRGINIANA LEAF WATER (UNII: 8FP93ED6H2) TEA TREE OIL (UNII: VIF565UC2G) PAPRIKA (UNII: X72Z47861V) PHENOXYETHANOL (UNII: HIE492ZZ3T) CAPRYLIC ALCOHOL (UNII: NV1779205D) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) HEXYLENE GLYCOL (UNII: KEH0A3F75J) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72380-728-10 1 in 1 BOX 12/01/2018 1 88.7 mL in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 12/01/2018 Labeler - Vivera Pharmaceuticals, Inc. (081244342)