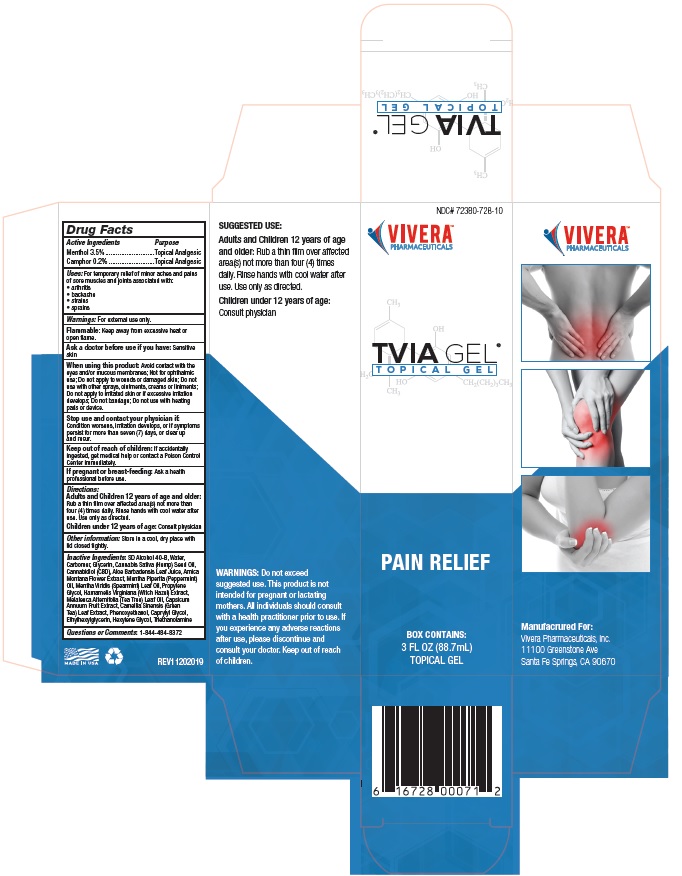
PURPOSE
Menthol ............................Topical Analgesic
Camphor ..........................Topical Analgesic
USES: For temporary relief from minor aches and
pains of sore muscles and joints associated with:
arthritis pain • backache • strains • sprains
When using this product: Avoid contact with the eyes
and/or mucous membranes • Do not use with other
sprays, ointments, creams or liniments • Do not apply
to irritated skin, & discontinue use if excessive skin
irritation develops • Do not bandage • Do not use
with heating pads or device.
Stop use and contact your physician if: conditions
worsen, or if symptoms persist for more than seven (7)
days, or clear up and reoccur.
If pregnant or breast-feeding: Ask a health
professional before use. Children under 2 years of age:
Consult physician
KEEP OUT OF REACH OF CHILDREN: If swallowed,
get medical help or contact a poison control center
immediately
DIRECTIONS: Use only as directed. Rub a thin film
over the affected areas not more than four (4) times
daily. Rinse hands after use with cool water.
INACTIVE INGREDIENTS: Ethyl Alcohol Denatured,
Deionized Water, Glycerin, Propylene Glycol, Cannabis
Sativa Seed Oil (Hemp Seed Oil), Cannabidiol (CBD), Arnica
Montana Flower Extract (Arnica Oil), Mentha Piperita
(Peppermint) Oil, Carbomer, Triethanolamine, Mentha Viridis
(Spearmint) Leaf Oil, Camellia Sinensis Leaf Extract, Aloe
Barbadensis Leaf Extract, Hamamelis Virginiana (Witch
Hazel) Extract, Melaleuca Alternifolia (Tea Tree) Leaf Oil,
Principal Display Package
NDC# 72380-728-10
VIVERA®
PHARMACEUTICALS
TVIA GEL®
T O P I C A L G E L
PAIN RELIEF
BOX CONTAINS:
3 FL OZ (88.7mL)
TOPICAL GEL
6 16728 00071 2
TVIA GEL®
T O P I C A L G E L
SUGGESTED USE: Apply a
small amount topically to
affected area and rub in. Use
every 4 to 6 hours. Safe for
use on adults over 21 years
of age. Wash hands with cool
water after each use.
WARNINGS: Do not exceed
suggested use. This product
is not intended for pregnant
or lactating mothers. All
individuals should consult with
a health practitioner prior
to use. If you experience any
adverse reactions after use,
please discontinue and consult
your doctor. Keep out of reach
of children. This product has
not been evaluated by the
Food and Drug Administration.
VIVERA®
PHARMACEUTICALS
*These statements have not been
evaluated by the Food and Drug
Administration. This product is not
intended to diagnose, treat, cure or
prevent any disease.
VIV-71000-GBX REV20190115
VIVERA®
PHARMACEUTICALS
Manufacrured For:
Vivera Pharmaceuticals, Inc.
11100 Greenstone Ave
Santa Fe Springs, CA 90670
MADE IN USA
Retail Box
Tube

Insert

TVIA Topical Gel by Vivera Pharmaceuticals, Inc.
res