Label: VELPHORO- sucroferric oxyhydroxide tablet, chewable
- NDC Code(s): 49230-645-51
- Packager: Fresenius Medical Care North America
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated July 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use VELPHORO ®safely and effectively. See full prescribing information for VELPHORO ®.
VELPHORO ®(sucroferric oxyhydroxide) chewable tablet for oral use
Initial U.S. Approval: 2013INDICATIONS AND USAGE
Velphoro is a phosphate binder indicated for the control of serum phosphorus levels in adult and pediatric patients 9 years of age and older with chronic kidney disease on dialysis. ( 1)
DOSAGE AND ADMINISTRATION
- Chew or crush Velphoro tablets, do not swallow whole. ( 2.2)
- The recommended starting dose for adults and pediatric patients 12 years of age and older is one 500 mg tablet three times daily with meals. ( 2.1)
- The recommended starting dose for pediatric patients 9 to <12 years of age is one 500 mg tablet two times daily with meals. ( 2.1)
- Adjust dosage by one 500 mg tablet per day as needed until an acceptable serum phosphorus level is reached, with regular monitoring afterwards. Titrate as often as weekly. ( 2.1)
DOSAGE FORMS AND STRENGTHS
- Velphoro chewable tablet 500 mg ( 3)
CONTRAINDICATIONS
- None ( 4)
WARNINGS AND PRECAUTIONS
- Patients with peritonitis during peritoneal dialysis, significant gastric or hepatic disorders, history of recent major gastrointestinal surgery, or with a history of hemochromatosis or other diseases with iron accumulation have not been included in clinical studies with Velphoro. Monitor effect and iron homeostasis in such patients. ( 5.1)
ADVERSE REACTIONS
- The most common adverse drug reactions to Velphoro chewable tablets in clinical trials were discolored feces (12%) and diarrhea (6%). ( 6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Fresenius Medical Care North America at 1-800-323-5188 or FDA at 1-800-FDA-1088 orwww.fda.gov/medwatch.
DRUG INTERACTIONS
- Velphoro can be administered concomitantly with oral calcitriol, ciprofloxacin, digoxin, enalapril, furosemide, HMG‑CoA reductase inhibitors, hydrochlorothiazide, losartan, metoprolol, nifedipine, omeprazole, quinidine and warfarin. ( 7)
- Take acetylsalicylic acid, cephalexin and doxycycline at least 1 hour before Velphoro. ( 7)
- Take levothyroxine at least 4 hours before Velphoro. ( 7)
- For oral medications not listed above where a reduction of bioavailability would be clinically significant consider separation of the timing of administration. Consider monitoring clinical responses or blood levels of the concomitant medication ( 7)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 7/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 General Dosing Information
2.2 Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Monitoring in Patients with Gastrointestinal Disorders or Iron Accumulation Disorders
6 ADVERSE REACTIONS
6.1 Clinical Trial Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Fixed-dose Study
14.2 Dose Titration Study
14.3 Pediatric Study
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 General Dosing Information
The recommended starting dose of Velphoro in adults and pediatric patients 12 years of age and older is one 500 mg tablet three times daily with meals. The recommended starting dose of Velphoro in pediatric patients 9 to <12 years of age is one 500 mg tablet two times daily with meals.
Monitor serum phosphorus levels and titrate the dose of Velphoro in decrements or increments of one 500 mg tablet per day as needed until an acceptable serum phosphorus level is reached, with regular monitoring afterwards. Titrate as often as weekly. If the recommended daily dose cannot be divided equally among meals, administer the larger dose with the largest meal of the day.
In clinical studies, on average, adult patients and pediatric patients 12 years of age and older required 1,500 mg to 2,000 mg (3 to 4 tablets) a day to control serum phosphorus levels; pediatric patients 9 to <12 years of age required on average 1,500 mg (3 tablets) to control serum phosphorus levels. Daily doses as high as 3,000 mg per day have been studied in adults and pediatric patients 9 years of age and older.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Monitoring in Patients with Gastrointestinal Disorders or Iron Accumulation Disorders
Each chewable tablet contains 500 mg iron (equivalent to 2,500 mg sucroferric oxyhydroxide) [see Clinical Pharmacology ( 12.3)] . Patients with peritonitis during peritoneal dialysis, significant gastric or hepatic disorders, following major gastrointestinal (GI) surgery, or with a history of hemochromatosis or other diseases with iron accumulation have not been included in clinical studies with Velphoro.
Monitor effect and iron homeostasis in such patients.
-
6 ADVERSE REACTIONS
6.1 Clinical Trial Experience
Adult Patients
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety data derived from Velphoro clinical trials reflect exposure to Velphoro in 2 active-controlled clinical studies involving a total of 778 adult patients on hemodialysis and 57 adult patients on peritoneal dialysis exposed for up to 55 weeks. Dosage regimens ranged from 250 mg to 3,000 mg per day.
In a parallel design, dose-finding study of Velphoro with a treatment duration of 6 weeks in hemodialysis patients, adverse reactions for Velphoro (N=128) were similar to those reported for the active-control group (sevelamer hydrochloride) (N=26), with the exception of discolored feces (12%) which did not occur in the active-control group. Diarrhea was reported in 6% of patients treated with Velphoro. In a 55-week, open-label, active-controlled, parallel design, safety and efficacy study involving 968 hemodialysis patients and 86 peritoneal dialysis patients treated with either Velphoro (N=707 including 57 peritoneal dialysis patients) or the active-control (sevelamer carbonate) (N=348 including 29 peritoneal dialysis patients), adverse reactions occurring in more than 5% in the Velphoro group were diarrhea (24%), discolored feces (16%), and nausea (10%). The majority of diarrhea events in the Velphoro group were mild and transient, occurring soon after initiation of treatment, and resolving with continued treatment. Adverse reactions occurred at similar rates in hemodialysis and peritoneal dialysis patients. The most common adverse reactions (>1%) leading to withdrawal were diarrhea (4%), product taste abnormal (2%), and nausea (2%).
Pediatric Patients
In an open-label, randomized study, with a 10-week dose titration period and 24-week safety extension, 60 patients 6 to 18 years of age received at least one dose of Velphoro, including 30 patients (50%) exposed for at least 19 weeks [see Clinical Studies ( 14.3)] . The safety profile of Velphoro in pediatric patients was similar to that observed in adult patients. Velphoro is not approved in pediatric patients 6 years to less than 9 years of age because of the lack of an appropriate dosage strength.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of Velphoro that are not included in other sections of labeling. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Gastrointestinal Disorders: tooth discoloration
Skin and Subcutaneous Tissue Disorders: rash
-
7 DRUG INTERACTIONS
Table 1 Oral drugs that can be administered concomitantly with Velphoro
Calcitriol
Ciprofloxacin
Digoxin
Enalapril
Furosemide
HMG-CoA reductase inhibitors
Hydrochlorothiazide
Losartan
Metoprolol
Nifedipine
Omeprazole
Quinidine
Warfarin
Oral drugs that are to be separated from Velphoro
Dosing Recommendations Doxycycline
Acetylsalicylic acid CephalexinTake at least 1 hour before Velphoro.
Levothyroxine Take at least 4 hours before Velphoro Oral medications not listed in Table 1
There are no empirical data on avoiding drug interactions between Velphoro and most concomitant oral drugs. For oral medications where a reduction in the bioavailability of that medication would have a clinically significant effect on its safety or efficacy, consider separating the administration of the two drugs. The necessary separation depends upon the absorption characteristics of the medication concomitantly administered, such as the time to reach peak systemic levels and whether the drug is an immediate-release or an extended-release product. Where possible, consider monitoring for clinical response and/or blood levels of concomitant medications that have a narrow therapeutic range.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Velphoro is not systemically absorbed following oral administration and maternal use is not expected to result in fetal exposure to the drug.
Data
Animal Data
In pregnant rats given up to 800 mg/kg/day Velphoro by oral gavage from Days 6 to 17 post-mating, no embryo-fetal development toxicity was observed. This dose corresponds to 16 times the maximum recommended clinical dose.
In pregnant rabbits given 50, 100 or 200 mg/kg/day Velphoro by oral gavage, from Days 6 to 19 post-mating, the number of fetuses with incomplete/unossified epiphyses and metacarpals/phalanges was increased at the highest dose (corresponding to 4 times the recommended maximum clinical dose). Litter parameters were not adversely affected.
In pregnant rats given Velphoro at 100, 280, or 800 mg/kg/day by oral gavage from Day 6 post-mating to lactation Day 20, offspring body weight gain was lower at age 5-13 weeks and neuromuscular function was delayed at the dose of 800 mg/kg/day. This dose represented 16 times the maximum recommended clinical dose.
8.2 Lactation
Velphoro is not absorbed systemically following oral administration and breastfeeding is not expected to result in exposure of the child to Velphoro.
8.4 Pediatric Use
Velphoro is indicated for the control of serum phosphorus levels in pediatric patients 9 years of age and older with CKD on dialysis. Use of Velphoro for this indication is supported by evidence from an adequate and well-controlled study in adults, with additional pharmacodynamic and safety data in pediatric patients with advanced CKD (an estimated glomerular filtration rate <30 mL/min/1.73 m 2or CKD on dialysis) [see Adverse Reactions ( 6.1) and Clinical Studies ( 14.3)] .
The safety and effectiveness of Velphoro have been established for the control of serum phosphorus levels in pediatric patients 6 years to less than 9 years of age with CKD on dialysis, but Velphoro is not approved in pediatric patients 6 years to less than 9 years of age because of the lack of an appropriate dosage strength.
The safety and effectiveness of Velphoro have not been established in pediatric patients younger than 6 years of age. Although the study included six patients 2 to <6 years of age who received Velphoro, based on the available data, it is unclear whether the dosing regimen that was evaluated is effective in reducing serum phosphorus in this age group.
Velphoro has not been studied in pediatric patients below 2 years of age.
8.5 Geriatric Use
Of the total number of subjects in two active-controlled clinical studies of Velphoro (N=835), 29.7% (n=248) were 65 and over, while 8.7% (n=73) were aged 75 years and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects.
-
10 OVERDOSAGE
There are no reports of overdosage with Velphoro in patients. Since the absorption of iron from Velphoro is low [see Clinical Pharmacology ( 12.3)] , the risk of systemic iron toxicity is low. Hypophosphatemia should be treated by standard clinical practice.
Velphoro has been studied in doses up to 3,000 mg per day.
-
11 DESCRIPTION
The Velphoro drug substance is a mixture of polynuclear iron(III)-oxyhydroxide, sucrose, and starches. The active moiety of Velphoro, polynuclear iron(III)-oxyhydroxide, is practically insoluble and cannot be absorbed.
Velphoro chewable tablets for oral use are brown, circular, bi-planar, and are embossed with “PA 500” on 1 side. Each tablet of Velphoro contains 500 mg iron (in 2,500 mg sucroferric oxyhydroxide). One tablet is equivalent to approximately 1.4 g of carbohydrates (750 mg sucrose and 700 mg starches as potato starch and pregelatinized maize starch). The inactive ingredients are berry flavor, neohesperidin dihydrochalcone, magnesium stearate, and silica (colloidal, anhydrous).
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
In the aqueous environment of the GI tract, phosphate binding takes place by ligand exchange between hydroxyl groups and/or water in sucroferric oxyhydroxide and the phosphate in the diet. The bound phosphate is eliminated with feces.
Both serum phosphorus levels and calcium-phosphorus product levels are reduced as a consequence of the reduced dietary phosphate absorption.
12.2 Pharmacodynamics
In vitrostudies have demonstrated a robust phosphate binding capacity of Velphoro over the physiologically relevant pH range of the GI tract (1.2-7.5). The phosphate binding capacity of Velphoro peaked at pH 2.5, resulting in 96% of the available phosphate being adsorbed (phosphorus:iron concentration ratio 0.4:1).
12.3 Pharmacokinetics
The active moiety of Velphoro, polynuclear iron (III)-oxyhydroxide (pn-FeOOH), is practically insoluble in water and therefore not absorbed and not metabolized. Its degradation product, mononuclear iron species, can however be released from the surface of pn-FeOOH and be absorbed.
Because of the insolubility and degradation characteristics of Velphoro, no classical pharmacokinetic studies can be carried out.
The sucrose and starch components of Velphoro can be digested to glucose and fructose, and maltose and glucose, respectively. These compounds can be absorbed in the blood.
The iron uptake from radiolabeled Velphoro drug substance, 2,000 mg in 1 day, was investigated in 16 chronic kidney disease patients (8 pre-dialysis and 8 hemodialysis patients) and 8 healthy volunteers with low iron stores (serum ferritin <100 mcg/L). In healthy subjects, the median uptake of radiolabeled iron in the blood was 0.43% on Day 21. In chronic kidney disease patients, the median uptake was much less, 0.04% on Day 21.
Drug Interaction Studies
In vitro
In vitrointeractions were studied in aqueous solutions which mimic the physico-chemical conditions of the gastro-intestinal tract with or without the presence of phosphate (400 mg). The study was conducted at pH 3.0, 5.5 and 8.0 with incubation at 37°C for 6 hours.
Interaction with Velphoro was seen with the following drugs: alendronate, doxycycline, acetylsalicylic acid, cephalexin, levothyroxine, and paricalcitol.
The following drugs did not show interaction with Velphoro: ciprofloxacin, enalapril, hydrochlorothiazide, metoprolol, nifedipine, and quinidine.
In vivo
Five in vivodrug interaction studies (N=40/study) were conducted with losartan, furosemide, digoxin, omeprazole and warfarin in healthy subjects receiving 1,000 mg Velphoro 3 times a day with meals. Velphoro did not alter the systemic exposure as measured by the area under the curve (AUC) of the tested drugs when co-administered with Velphoro or given 2 hours later.
Data from the clinical studies (Study-05A and Study-05B) show that Velphoro does not affect the lipid lowering effects of HMG-CoA reductase inhibitors or the PTH lowering effect of calcitriol.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies were performed in mice and rats.
In the 2-year carcinogenicity study in mice, animals were given Velphoro by diet at doses of 250, 500 or 1,000 mg/kg/day. There was no clear evidence of a carcinogenic effect in mice. Mucosal hyperplasia, with diverticulum/cyst formation was observed in the colon and caecum of mice after 2 years treatment. In the 2-year rat carcinogenicity study, animals were given Velphoro by diet at doses of 40, 150 or 500 mg/kg/day. No statistically significantly increased incidences of tumors were found, but there were increased incidences in epithelial hyperplasia with or without submucosal inflammation in duodenum, cecum and colon at the dose of 500 mg/kg/day (10 times the maximum recommended clinical dose).
Velphoro was not mutagenic, clastogenic or DNA damaging in vitroin the Ames bacterial reverse mutation test, or in the Chinese-hamster fibroblast chromosomal aberration test, or in vivoin the rat Comet assay or peripheral blood micronucleus test.
In rats, mating performance and fertility were unaffected by Velphoro at oral doses up to 800 mg/kg/day (16 times the maximum recommended clinical dose).
-
14 CLINICAL STUDIES
14.1 Fixed-dose Study
In Study-03A, 154 ESRD adult patients on hemodialysis who were hyperphosphatemic (serum phosphorus >5.5 mg/dL but <7.75 mg/dL) following a 2-week phosphate binder washout period, were randomized to receive Velphoro at 250 mg/day, 1,000 mg/day, 1,500 mg/day, 2,000 mg/day, or 2,500 mg/day or active-control (sevelamer hydrochloride). Velphoro treatment was divided across meals, depending on dose. No dose titration was allowed. Within each of the groups, the serum phosphorus level at the end of treatment was compared to baseline value. Velphoro was shown to be efficacious (p≤0.016) for all doses except 250 mg/day. There were no patient-reported dose limiting treatment-emergent adverse events.
Mean changes in iron parameters (ferritin, transferrin saturation (TSAT) and transferrin) and vitamins (A, D, E and K) were generally not clinically meaningful and showed no apparent trends across the treatment groups. Velphoro had a similar GI adverse event profile [see Adverse Reactions ( 6.1)] to sevelamer hydrochloride, and no dose-dependent trend in GI events was observed.
14.2 Dose Titration Study
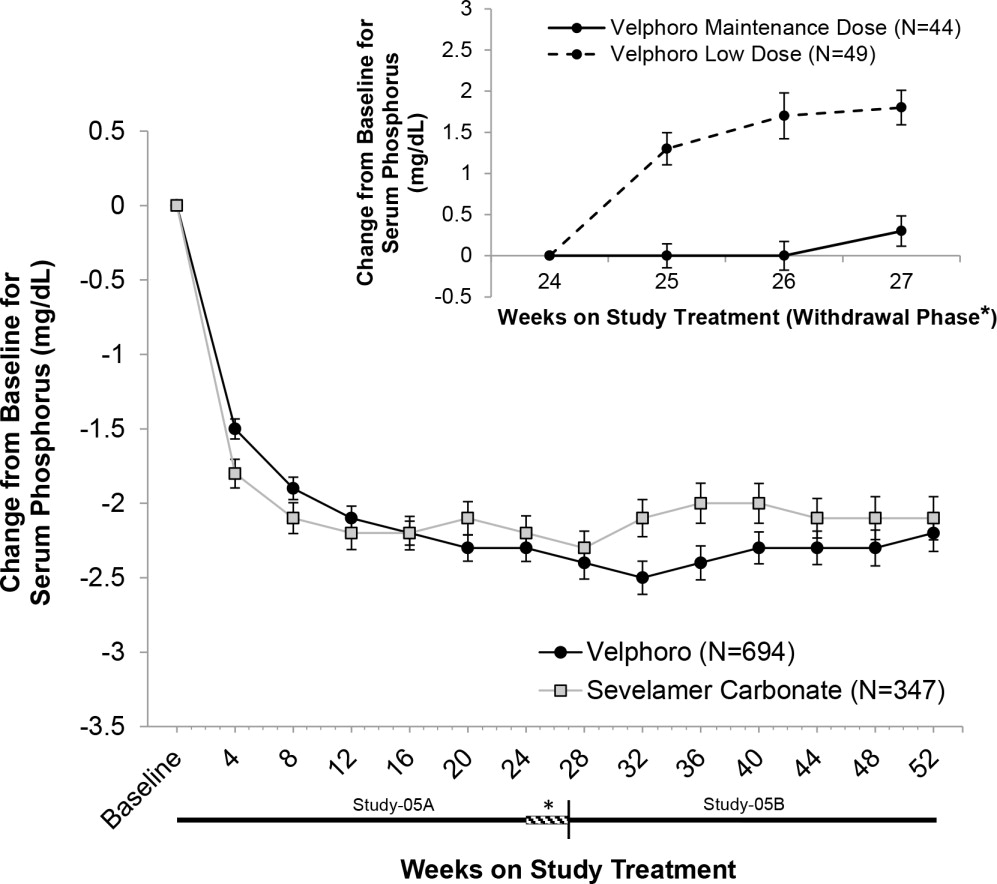
In Study-05A, 1,055 adult patients on hemodialysis (N=968) or peritoneal dialysis (N=87) with serum phosphorus ≥6 mg/dL following a 2-4 week phosphate binder washout period, were randomized and treated with either Velphoro, at a starting dose of 1,000 mg/day (N=707), or active-control (sevelamer carbonate, N=348) for 24 weeks. At the end of Week 24, 93 patients on hemodialysis whose serum phosphorus levels were controlled (<5.5 mg/dL) with Velphoro in the first part of the study, were re-randomized to continue treatment with either their Week 24 maintenance dose (N=44 or a non-effective low dose control 250 mg/day, N=49) of Velphoro for a further 3 weeks. At Week 27, a superiority analysis of the Velphoro maintenance dose versus low dose was performed. The maximum dose of Velphoro was 3,000 mg/day (6 tablets/day) and the minimum dose was 1,000 mg/day (2 tablets/day). Velphoro was administered with food and the daily dose was divided across the largest meals of the day.
The Velphoro maintenance dose (1,000 to 3,000 mg/day) was statistically significantly superior in sustaining the phosphorus lowering effect in hemodialysis patients at Week 27 (p<0.001) compared with the non-effective low dose control. The results are provided in Table 2.
Table 2 Mean (SD) Serum Phosphorus and Change from Baseline to End of Treatment * p<0.001 for the difference in least square means of the change from BL to Week 27/End of Treatment (LOCF principle) between Velphoro maintenance dose and low dose using a covariance analysis (MIXED Model).
Notes: BL is Week 24 or latest value available before Week 24 when Week 24 result is missing; End of Treatment is Week 27 value or includes the latest evaluable measurement after Week 24 (i.e., LOCF).
BL = Baseline; LOCF = Last observation carried forward; SD = Standard deviation.Mean (SD) Serum Phosphorus (mg/dL) Velphoro Maintenance Dose
(1,000 to 3,000 mg/day)
(N=44)Velphoro Low Dose Control
(250 mg/day)
(N=49)Week 24 (BL) 4.7 (1.03) 5.0 (1.14) Week 25 4.7 (0.91) 6.3 (1.44) Week 26 4.7 (1.21) 6.6 (1.91) Week 27/End of Treatment 5.0 (1.07) 6.8 (1.63) Change from BL to End of Treatment 0.3 (1.22)* 1.8 (1.47) Following completion of Study-05A, 658 patients (597 on hemodialysis and 61 on peritoneal dialysis) were treated in the 28-week extension study (Study-05B) with either Velphoro (N=391) or sevelamer carbonate (N=267) according to their original randomization.
Serum phosphorus levels declined rapidly during the first few weeks of treatment and remained relatively constant thereafter. The phosphorus lowering effect of Velphoro was consistently maintained and controlled through 12 months of treatment (shown in Figure 1). The proportion of adherent patients for Velphoro was 86% at 52 weeks.
Figure 1Mean change (±SEM) from baseline in serum phosphorus over time in Study‑05A and extension Study‑05B. Insert showing the mean change (±SEM) from baseline in serum phosphorus during the withdrawal phase of the study (Weeks 24 to 27) for Velphoro non-effective low dose control (250 mg/day) versus Velphoro maintenance dose.
Age, gender, race, or dialysis modality did not affect the efficacy of Velphoro.
There were no clinically meaningful changes for serum iron, ferritin, TSAT levels, vitamins (A, D, E and K) with Velphoro. There was no evidence of accumulation of iron during one year treatment.
14.3 Pediatric Study
A clinical study of Velphoro (NCT02688764) was conducted in pediatric patients with hyperphosphatemia and advanced CKD (defined as an estimated glomerular filtration rate <30 mL/min/l.73m 2or CKD on dialysis). Following a washout period of up to 3 weeks, 78 patients 6 to 18 years of age were randomized to Velphoro (N=60) or active control calcium acetate (Phoslyra) (N=18) for a 10-week dose titration period, followed by a 24-week safety extension. Approximately 81% of patients were CKD patients on dialysis (69% on hemodialysis and 12% on peritoneal dialysis). 43 patients randomized to Velphoro completed the dose titration period.
The least squares (LS) mean reduction in serum phosphorus levels from baseline to the end of the dose titration period in the Velphoro group (N=59) was -0.52 mg/dL (95% CI: -0.97, -0.07). The percentage of patients with serum phosphorus levels within normal ranges increased from 39% at baseline to 64% at the end of the dose titration period.
Velphoro is not approved in pediatric patients 6 years to less than 9 years of age because of the lack of an appropriate dosage strength.
The median prescribed daily dose of Velphoro was approximately 1500 mg during the dose titration period for patients 9 to 18 years of age.
- 16 HOW SUPPLIED/STORAGE AND HANDLING
-
17 PATIENT COUNSELING INFORMATION
Inform patients that Velphoro tablets should be chewed or crushed. Do not swallow whole [see Dosage and Administration ( 2.2)].
Advise patients that Velphoro should be taken with meals.
Instruct patients on concomitant medications that should be dosed apart from Velphoro [see Drug Interactions ( 7)].
Inform patients that Velphoro can cause discolored (black) stool, but this discoloration of the stool is considered normal with oral medications containing iron.
Inform patients that Velphoro can stain teeth.
Inform patients to report any rash to their health care professional.
Distributed by:
Fresenius Medical Care North America
920 Winter Street
Waltham, MA 02451
Patents Apply – visit www.fmcna.com/patents© 2014-2020, Fresenius Medical Care North America. All Rights Reserved.
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
VELPHORO
sucroferric oxyhydroxide tablet, chewableProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:49230-645 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FERRIC OXYHYDROXIDE (UNII: 87PZU03K0K) (FERRIC OXYHYDROXIDE - UNII:87PZU03K0K) IRON 500 mg Product Characteristics Color brown Score no score Shape ROUND Size 20mm Flavor BERRY Imprint Code PA;500 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49230-645-51 1 in 1 CARTON 11/27/2013 1 90 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA205109 11/27/2013 Labeler - Fresenius Medical Care North America (958291411) Registrant - Vifor Fresenius Medical Care Renal Pharma France (264470157)