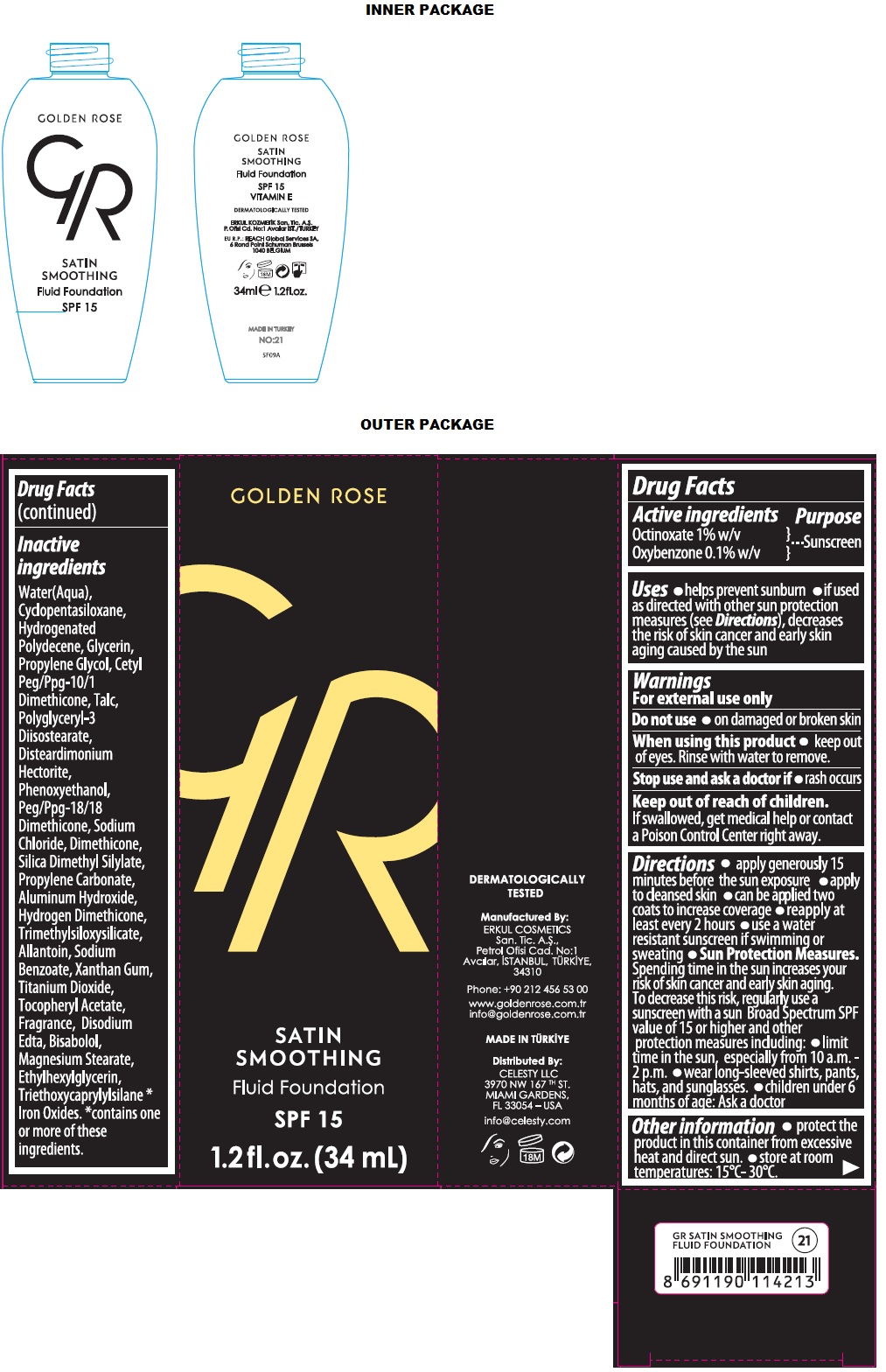
Label: GR SATIN SMOOTHING FLUID FOUNDATION SPF 15- octinoxate, oxybenzone liquid
-
NDC Code(s):
82715-104-01,
82715-104-02,
82715-104-03,
82715-104-04, view more82715-104-05, 82715-104-06, 82715-104-07, 82715-104-08, 82715-104-09
- Packager: ERKUL KOZMETIK SANAYI VE TICARET ANONIM SIRKETI
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated July 25, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
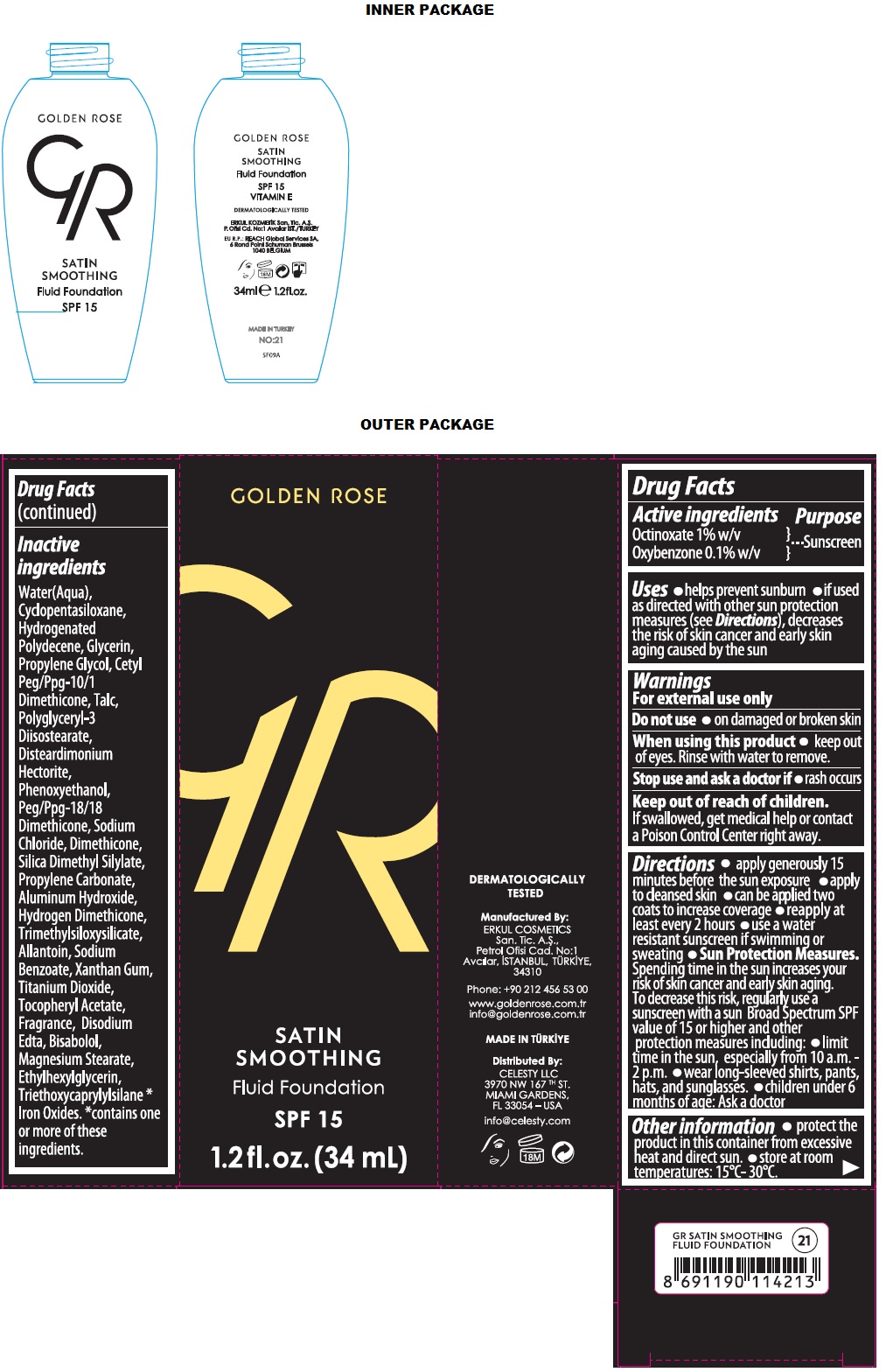
- Drug Facts
- Active ingredients
- Purpose
- Uses
- Warnings
-
Directions
• apply generously 15 minutes before the sun exposure • apply to cleansed skin • can be applied two coats to increase coverage • reapply at least every 2 hours • use a water resistant sunscreen if swimming or sweating • Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a sun Broad Spectrum SPF value of 15 or higher and other protection measures including: • limit time in the sun, especially from 10 a.m. - 2 p.m. • wear long-sleeved shirts, pants, hats, and sunglasses. • children under 6 months of age: Ask a doctor
- Other information
-
Inactive ingredients
Water(Aqua), Cyclopentasiloxane, Hydrogenated Polydecene, Glycerin, Propylene Gylcol, Cetyl Peg/Ppg-10/1 Dimethicone, Talc, Polyglyceryl-3 Diisostearate, Disteardimonium Hectorite, Phenoxyethanol, Peg/Ppg-18/18 Dimethicone, Sodium Chloride, Dimethicone, Silica Dimethyl Silylate, Propylene Carbonate, Aluminum Hydroxide, Hydrogen Dimethicone, Trimethylsiloxysilicate, Allantoin, Sodium Benzoate, Xanthan gum, Titanium Dioxide, Tocopheryl Acetate, Fragrance, Disodium Edta, Bisabolol, Magnesium Stearate, Ethylhexylglycerin, Triethoxycaprylylsilane *Iron Oxides. *contains one or more of these ingredients.
-
SPL UNCLASSIFIED SECTION
GOLDEN ROSE
SPF 15
VITAMIN E
DERMATOLOGICALLY TESTED
Manufactured By:
ERKUL COSMETICS San. Tic. A.Ş.,
Petrol Ofisi Cad. No:1
Avcılar, ISTANBUL, TURKIYE,
34310Phone: +90 212 456 53 00
www.goldenrose.com.tr
info@goldenrose.com.trMADE IN TURKIYE
Distributed By:
CELESTY LLC
3970 NW 167TH ST.
MIAMI GARDENS,
FL 33054 – USAinfo@celesty.com
EU R.P.: REACH Global Services SA,
6 Rond point Schuman Brussels
1040 BELGIUMNO:21
- Packaging
-
INGREDIENTS AND APPEARANCE
GR SATIN SMOOTHING FLUID FOUNDATION SPF 15
octinoxate, oxybenzone liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82715-104 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 1 g in 100 mL OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 0.1 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) HYDROGENATED POLYDECENE (3100 CST) (UNII: 4U179ML4TJ) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 4) (UNII: 8INO2K35FA) TALC (UNII: 7SEV7J4R1U) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) PHENOXYETHANOL (UNII: HIE492ZZ3T) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) SODIUM CHLORIDE (UNII: 451W47IQ8X) DIMETHICONE (UNII: 92RU3N3Y1O) SILICA DIMETHYL SILYLATE (UNII: EU2PSP0G0W) PROPYLENE CARBONATE (UNII: 8D08K3S51E) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) HYDROGEN DIMETHICONE (13 CST) (UNII: 4QGR4P2YOI) TRIMETHYLSILOXYSILICATE (M/Q 0.6-0.8) (UNII: 5041RX63GN) ALLANTOIN (UNII: 344S277G0Z) SODIUM BENZOATE (UNII: OJ245FE5EU) XANTHAN GUM (UNII: TTV12P4NEE) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) LEVOMENOL (UNII: 24WE03BX2T) MAGNESIUM STEARATE (UNII: 70097M6I30) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82715-104-01 1 in 1 CARTON 07/22/2022 1 34 mL in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:82715-104-02 1 in 1 CARTON 07/22/2022 2 34 mL in 1 BOTTLE; Type 0: Not a Combination Product 3 NDC:82715-104-03 1 in 1 CARTON 07/22/2022 3 34 mL in 1 BOTTLE; Type 0: Not a Combination Product 4 NDC:82715-104-04 1 in 1 CARTON 07/22/2022 4 34 mL in 1 BOTTLE; Type 0: Not a Combination Product 5 NDC:82715-104-05 1 in 1 CARTON 07/22/2022 5 34 mL in 1 BOTTLE; Type 0: Not a Combination Product 6 NDC:82715-104-06 1 in 1 CARTON 07/22/2022 6 34 mL in 1 BOTTLE; Type 0: Not a Combination Product 7 NDC:82715-104-07 1 in 1 CARTON 07/22/2022 7 34 mL in 1 BOTTLE; Type 0: Not a Combination Product 8 NDC:82715-104-08 1 in 1 CARTON 07/22/2022 8 34 mL in 1 BOTTLE; Type 0: Not a Combination Product 9 NDC:82715-104-09 1 in 1 CARTON 07/22/2022 9 34 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 07/22/2022 Labeler - ERKUL KOZMETIK SANAYI VE TICARET ANONIM SIRKETI (525225637) Establishment Name Address ID/FEI Business Operations ERKUL KOZMETIK SANAYI VE TICARET ANONIM SIRKETI 525225637 manufacture(82715-104)