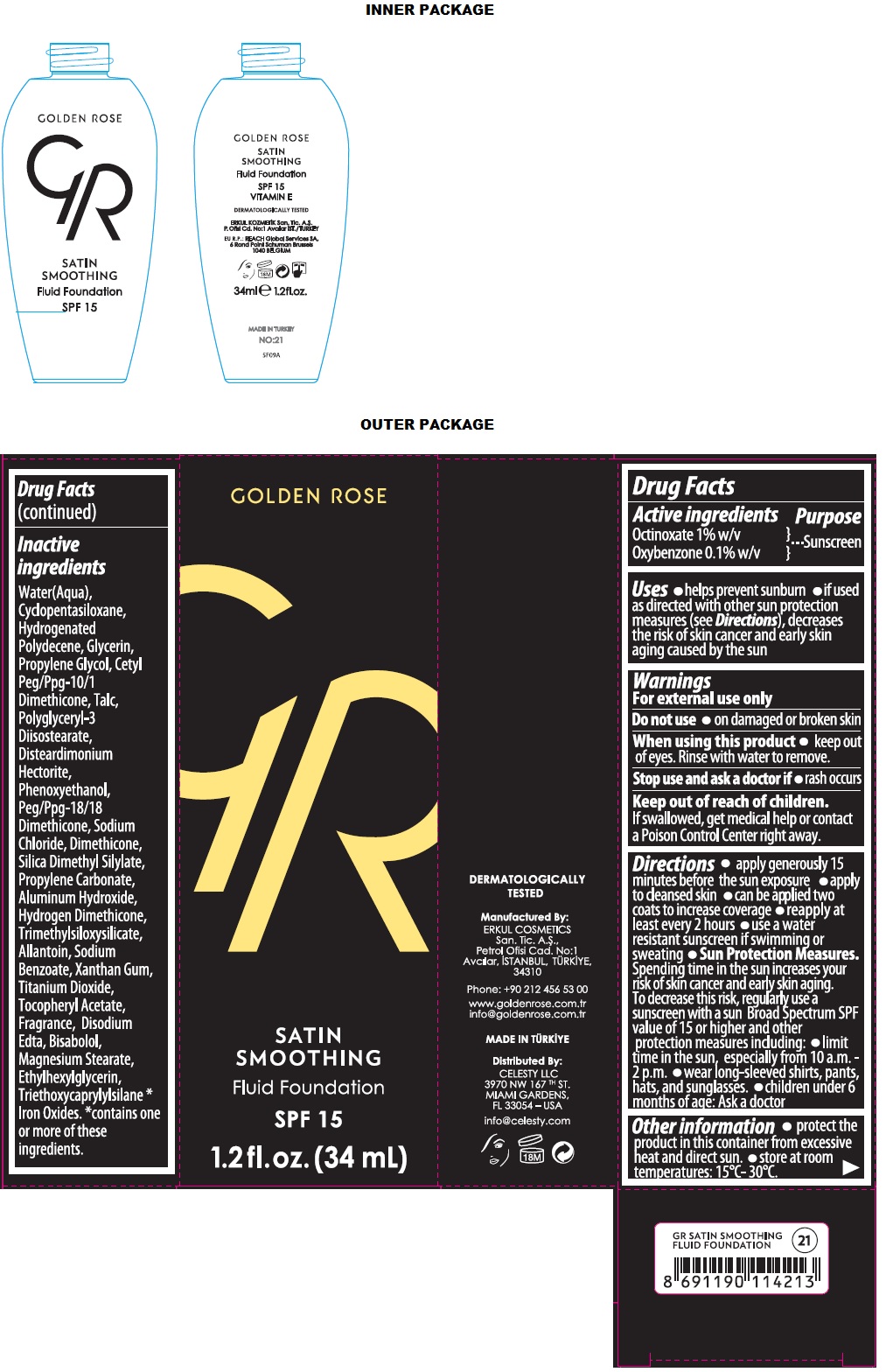
Uses
• helps prevent sunburn • if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
Warnings
For external use only
Do not use • on damaged or broken skin
When using this product • keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor if • rash occurs
Directions
• apply generously 15 minutes before the sun exposure • apply to cleansed skin • can be applied two coats to increase coverage • reapply at least every 2 hours • use a water resistant sunscreen if swimming or sweating • Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a sun Broad Spectrum SPF value of 15 or higher and other protection measures including: • limit time in the sun, especially from 10 a.m. - 2 p.m. • wear long-sleeved shirts, pants, hats, and sunglasses. • children under 6 months of age: Ask a doctor
Other information
• protect the product in this container from excessive heat and direct sun. • store at room temperatures: 15°C- 30°C.
Inactive ingredients
Water(Aqua), Cyclopentasiloxane, Hydrogenated Polydecene, Glycerin, Propylene Gylcol, Cetyl Peg/Ppg-10/1 Dimethicone, Talc, Polyglyceryl-3 Diisostearate, Disteardimonium Hectorite, Phenoxyethanol, Peg/Ppg-18/18 Dimethicone, Sodium Chloride, Dimethicone, Silica Dimethyl Silylate, Propylene Carbonate, Aluminum Hydroxide, Hydrogen Dimethicone, Trimethylsiloxysilicate, Allantoin, Sodium Benzoate, Xanthan gum, Titanium Dioxide, Tocopheryl Acetate, Fragrance, Disodium Edta, Bisabolol, Magnesium Stearate, Ethylhexylglycerin, Triethoxycaprylylsilane *Iron Oxides. *contains one or more of these ingredients.
GOLDEN ROSE
SPF 15
VITAMIN E
DERMATOLOGICALLY TESTED
Manufactured By:
ERKUL COSMETICS San. Tic. A.Ş.,
Petrol Ofisi Cad. No:1
Avcılar, ISTANBUL, TURKIYE,
34310
Phone: +90 212 456 53 00
www.goldenrose.com.tr
info@goldenrose.com.tr
MADE IN TURKIYE
Distributed By:
CELESTY LLC
3970 NW 167TH ST.
MIAMI GARDENS,
FL 33054 – USA
info@celesty.com
EU R.P.: REACH Global Services SA,
6 Rond point Schuman Brussels
1040 BELGIUM
NO:21