Label: NU SKIN NU COLOUR- octinoxate, octisalate, oxybenzone, and titanium dioxide lotion
NU SKIN NU COLOUR- octinoxate, octisalate, oxybenzone, and titanium dioxide lotion
NU SKIN NU COLOUR- octinoxate, octisalate, oxybenzone, and titanium dioxide lotion
NU SKIN NU COLOUR- octinoxate, octisalate, oxybenzone, and titanium dioxide lotion
NU SKIN NU COLOUR- octinoxate, octisalate, oxybenzone, and titanium dioxide lotion
NU SKIN NU COLOUR- octinoxate, octisalate, oxybenzone, and titanium dioxide lotion
NU SKIN NU COLOUR- octinoxate, octisalate, oxybenzone, and titanium dioxide lotion
NU SKIN NU COLOUR- octinoxate, octisalate, oxybenzone, and titanium dioxide lotion
NU SKIN NU COLOUR- octinoxate, octisalate, oxybenzone, and titanium dioxide lotion
NU SKIN NU COLOUR- octinoxate, octisalate, oxybenzone, and titanium dioxide lotion
-
Contains inactivated NDC Code(s)
NDC Code(s): 62839-0802-1, 62839-0803-1, 62839-0804-1, 62839-0805-1, view more62839-0806-1, 62839-0807-1, 62839-0808-1, 62839-0811-1, 62839-0812-1, 62839-0820-1 - Packager: NSE Products, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated June 3, 2011
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Warning
- Directions
-
Inactive Ingredients
Water (Aqua), Glycerin, Butylene Glycol, Propylene Glycol, Oleic Acid, Stearamide MEA, Cetyl Palmitate, Propylene Glycol Stearate, Retinyl Palmitate, Panthenol, Aloe Barbadensis Leaf Juice, Tocopheryl Acetate, Squalane, Jojoba Esters, Sodium PCA, Ascorbyl Palmitate, Bisabolol, Tocopherol, Zinc Glycinate, Silica, Dimethicone, Lecithin, Cetyl Alcohol, Magnesium Aluminum Silicate, Barium Sulfate, Polysorbate 20, Xanthan Gum, PEG-12 Dimethicone, Pyridoxine Dipalmitate, Simethicone, Disodium EDTA, Triethanolamine, Chlorphenesin, Methylparaben, Propylparaben.
- May Contain
- Questions?
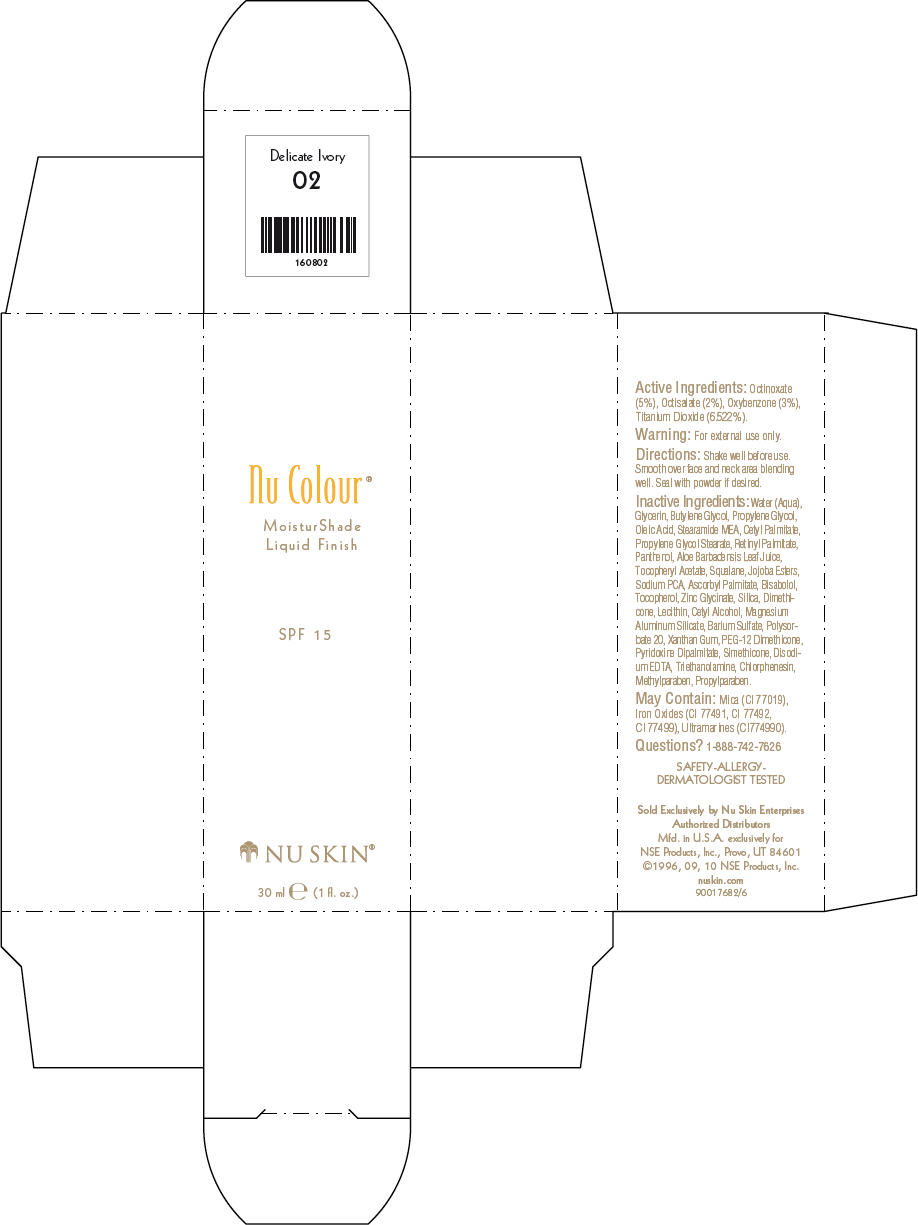
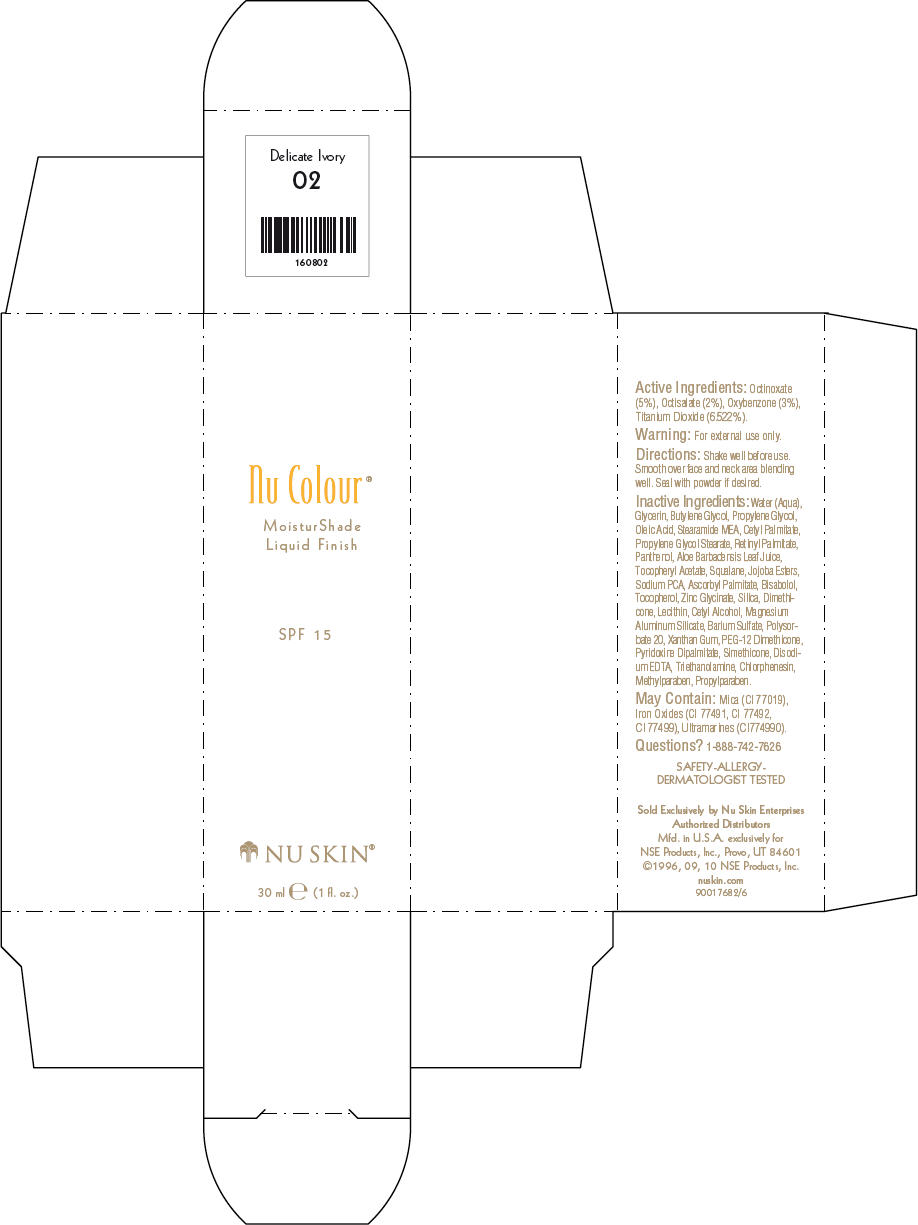
- PRINCIPAL DISPLAY PANEL - 30 ml Delicate Ivory Carton
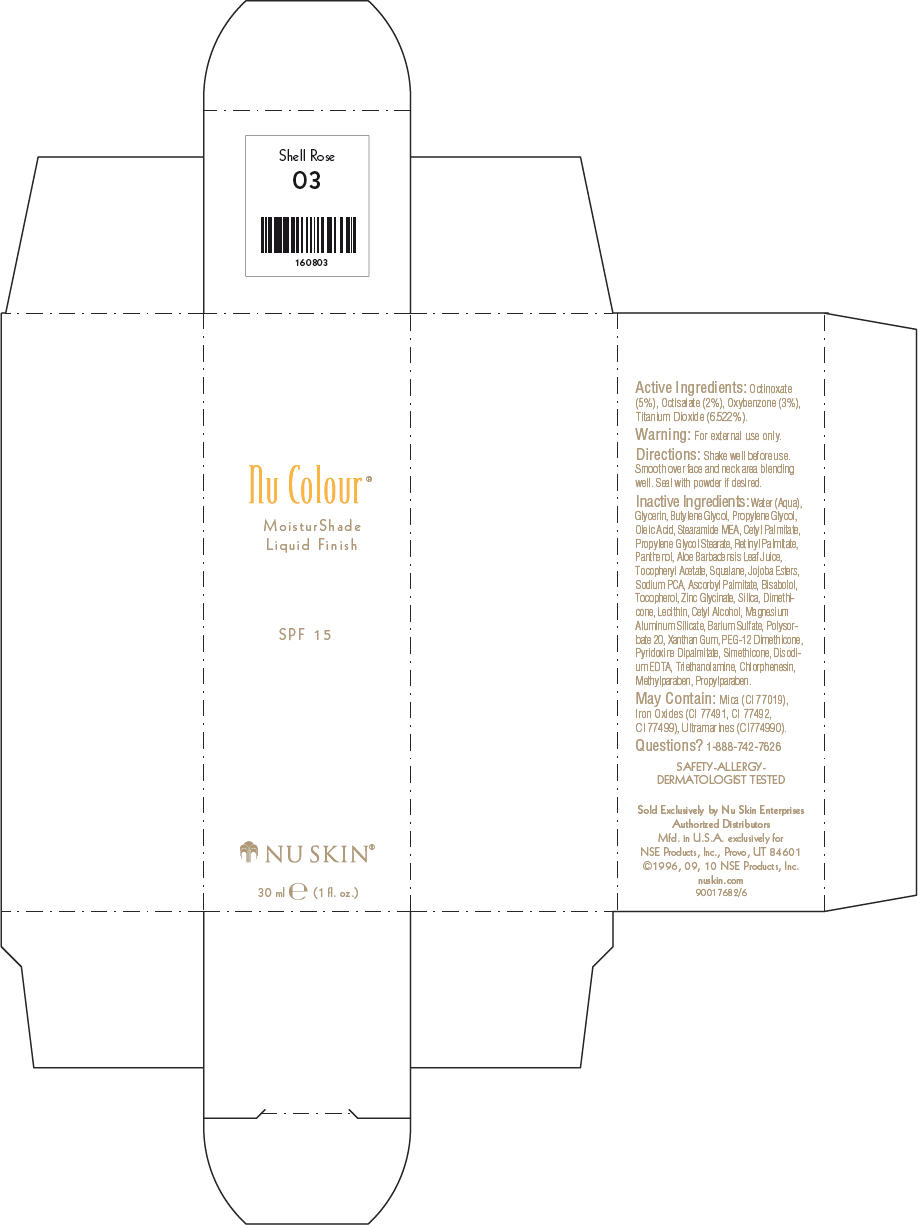
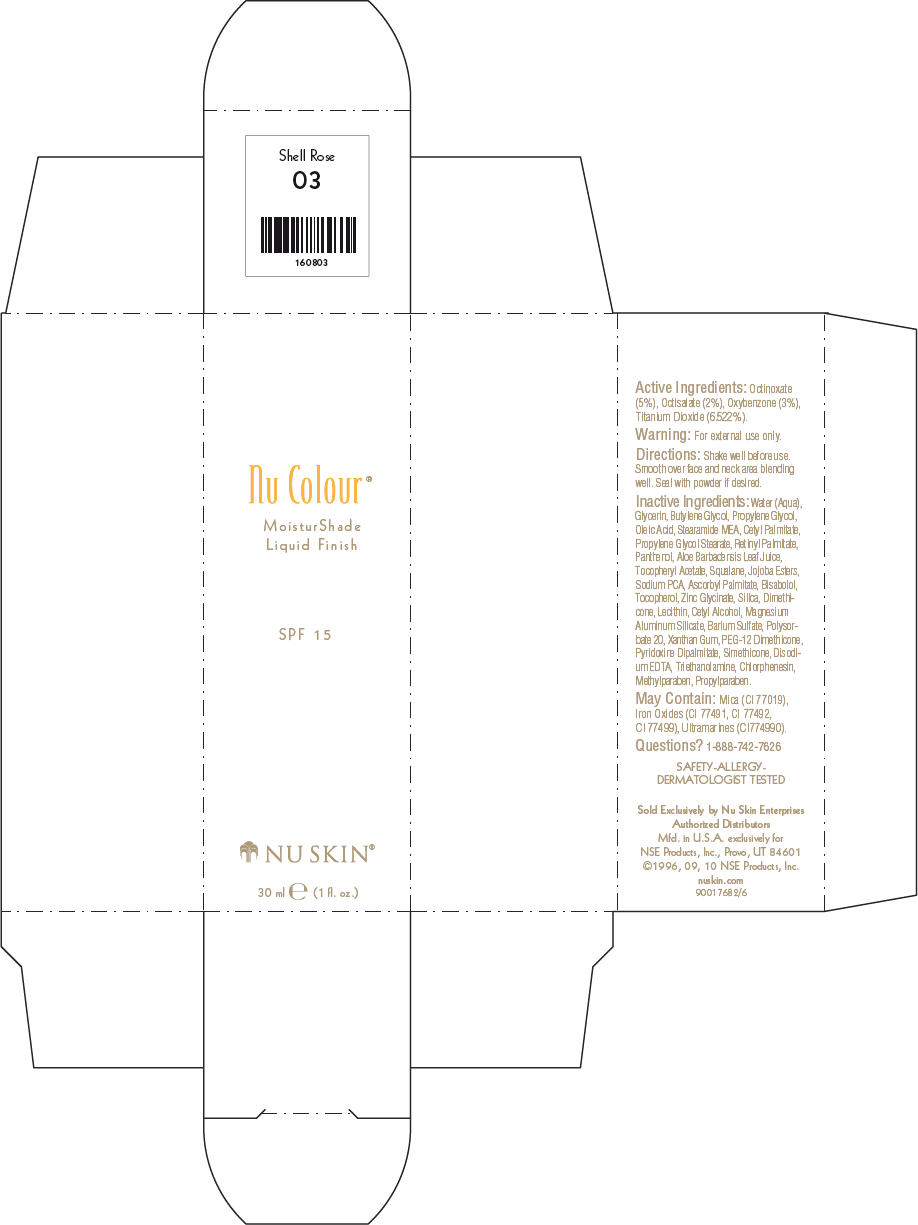
- PRINCIPAL DISPLAY PANEL - 30 ml Shell Rose Carton
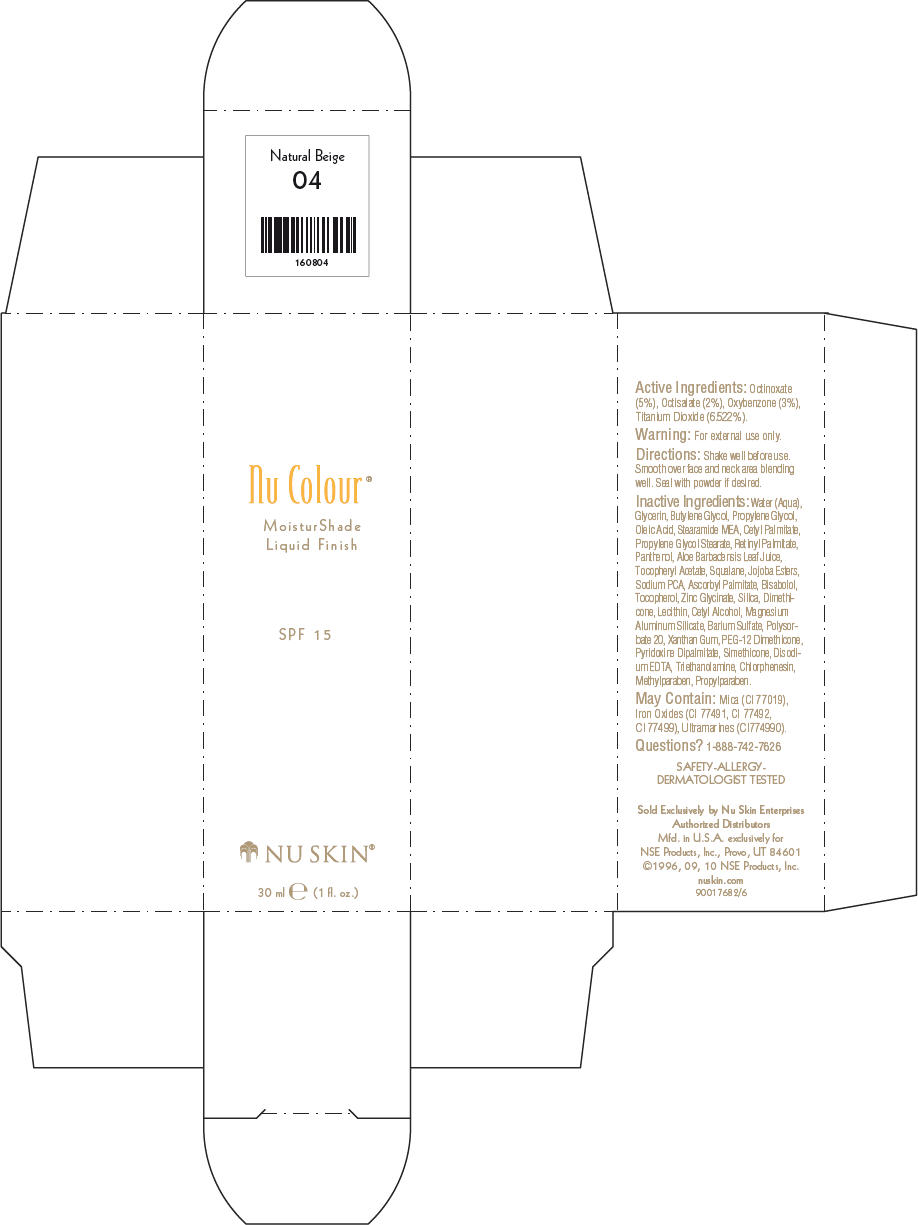
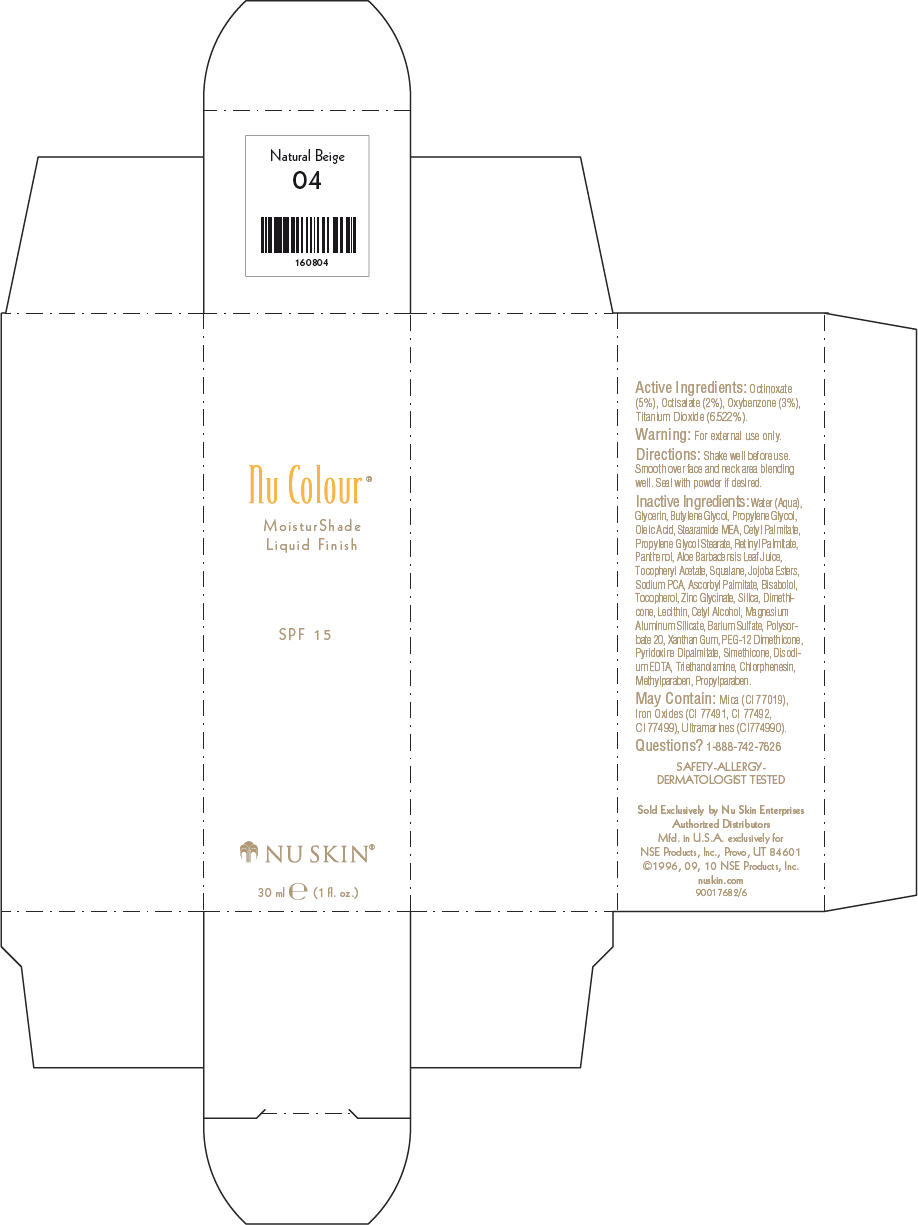
- PRINCIPAL DISPLAY PANEL - 30 ml Natural Beige Carton
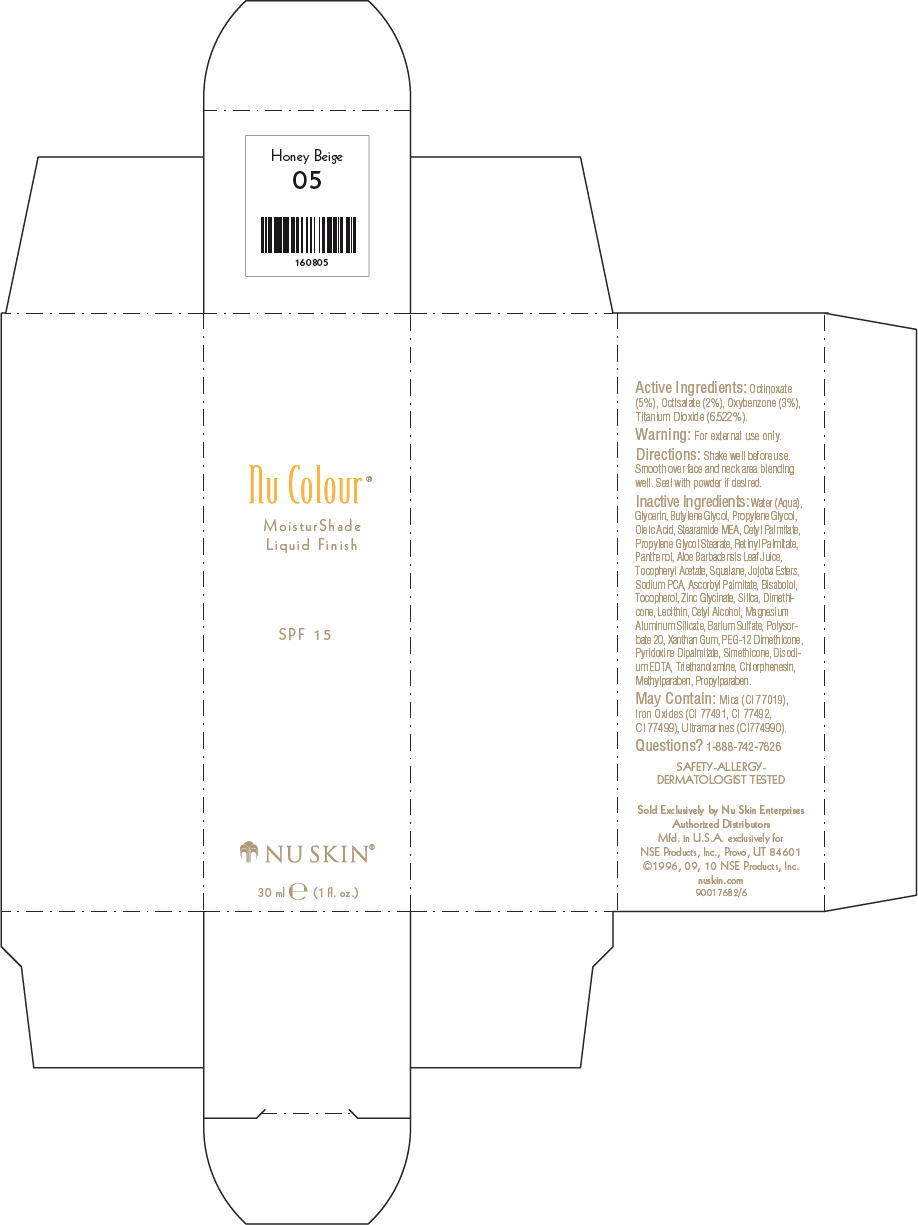
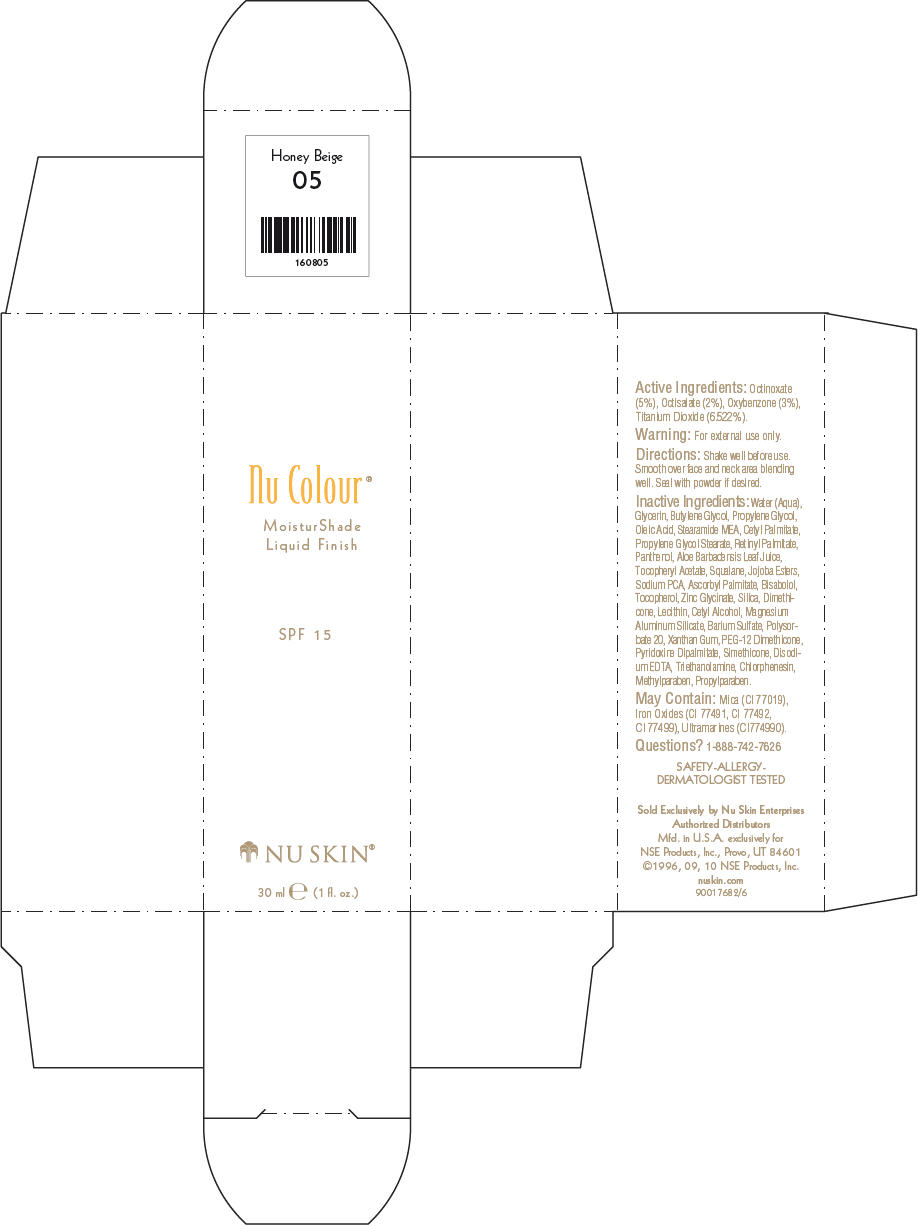
- PRINCIPAL DISPLAY PANEL - 30 ml Honey Beige Carton
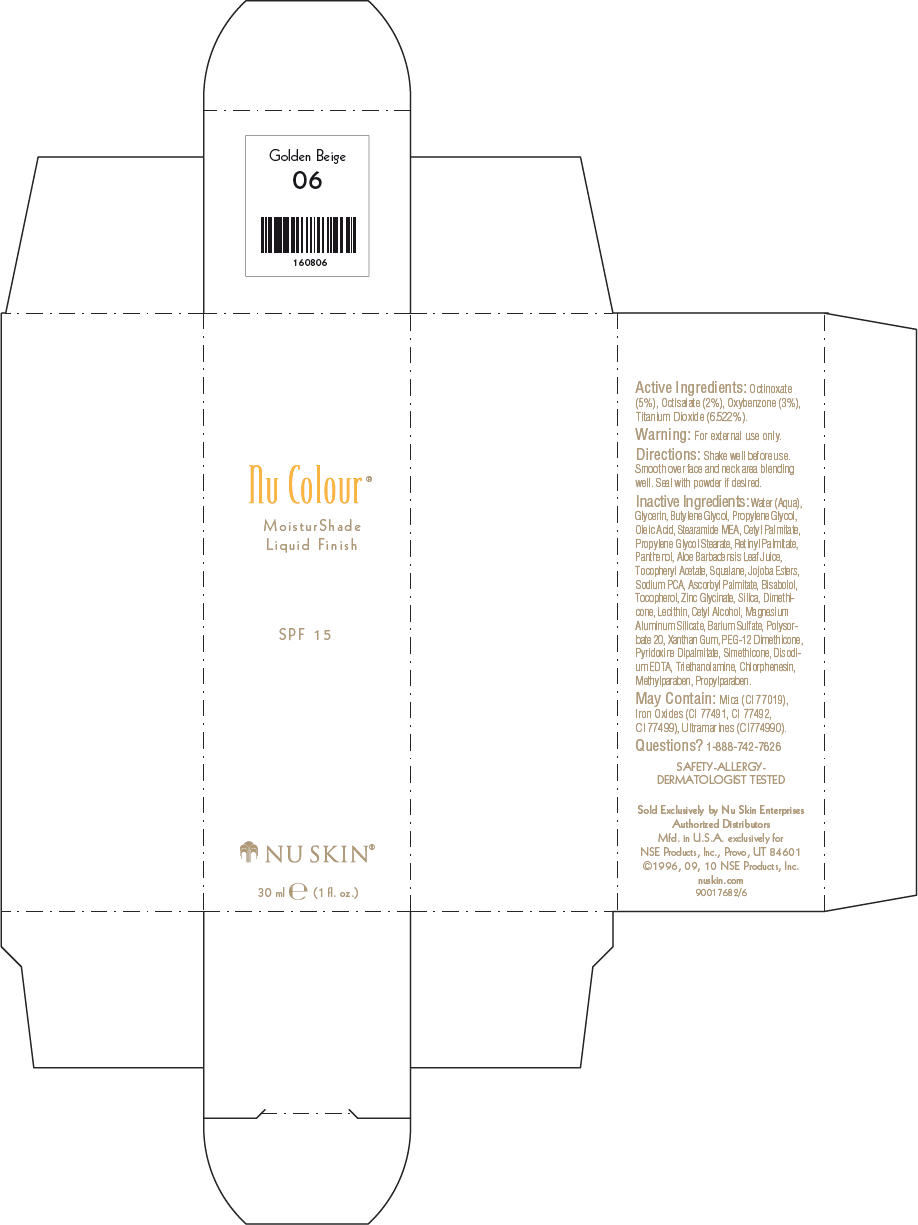
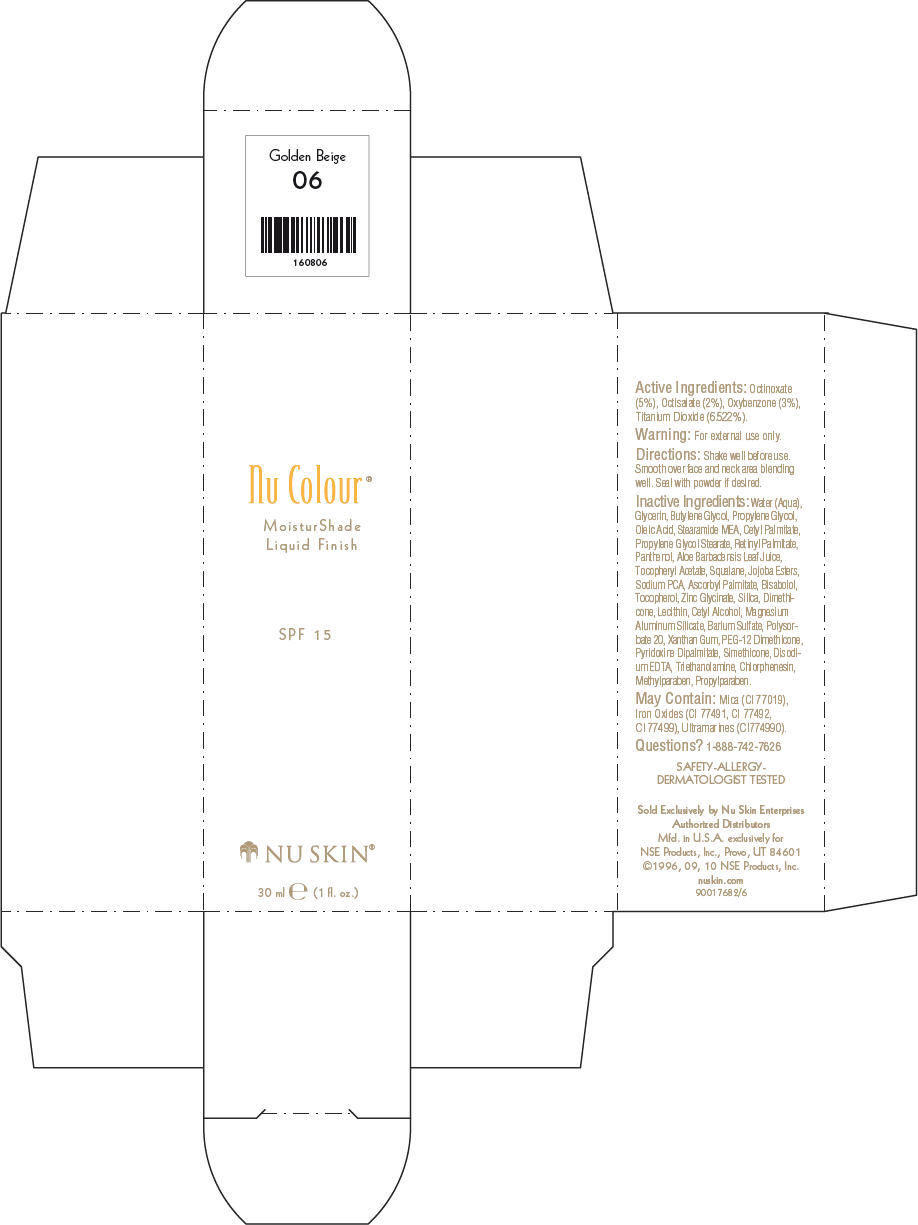
- PRINCIPAL DISPLAY PANEL - 30 ml Golden Beige Carton
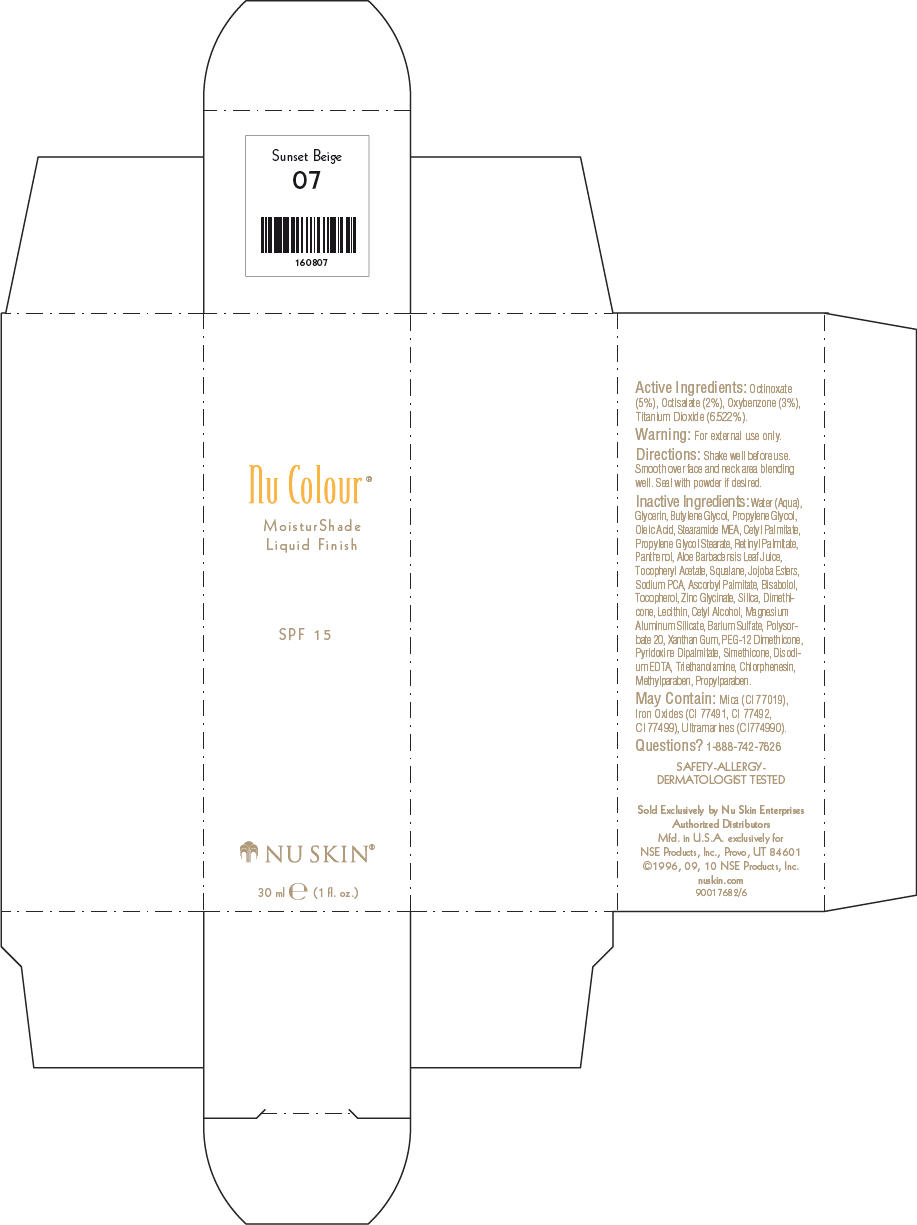
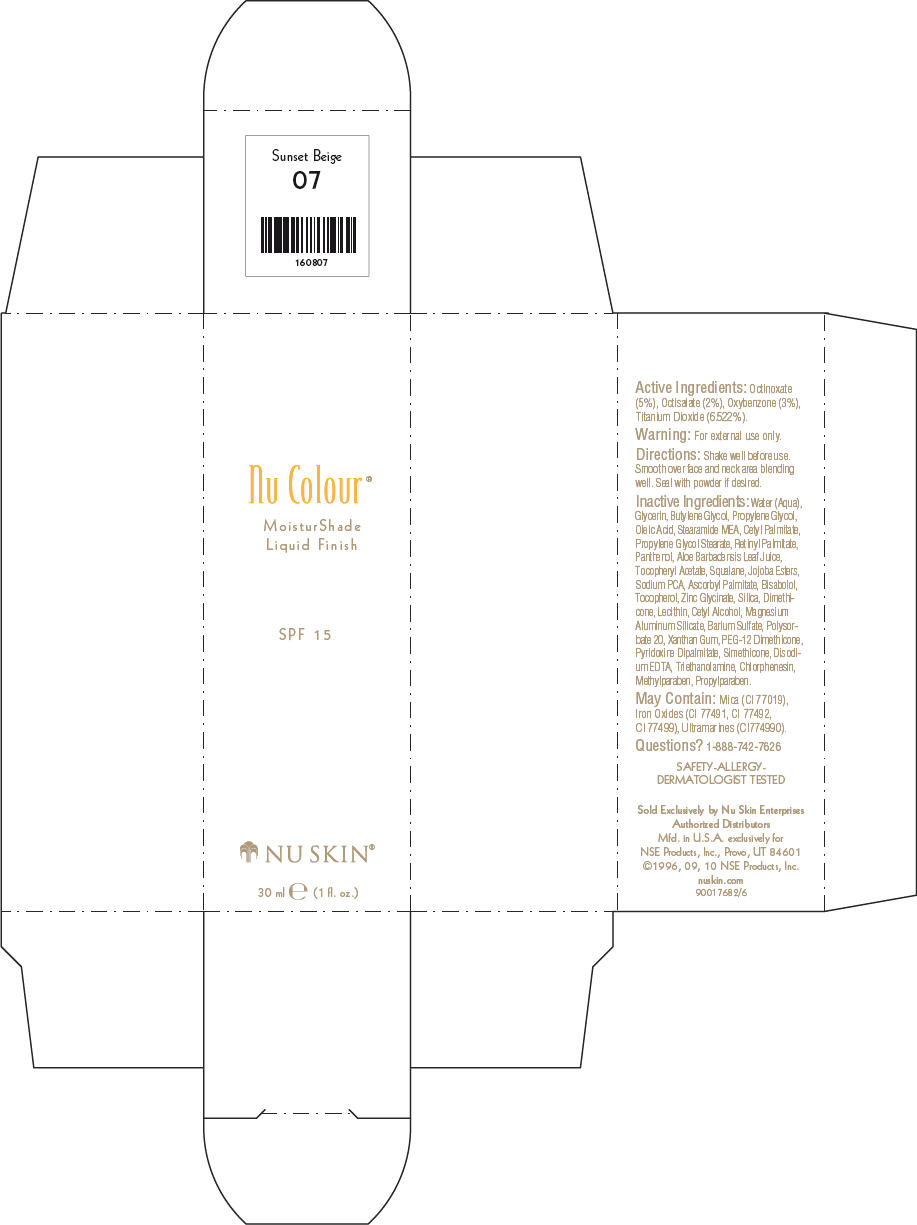
- PRINCIPAL DISPLAY PANEL - 30 ml Sunset Beige Carton
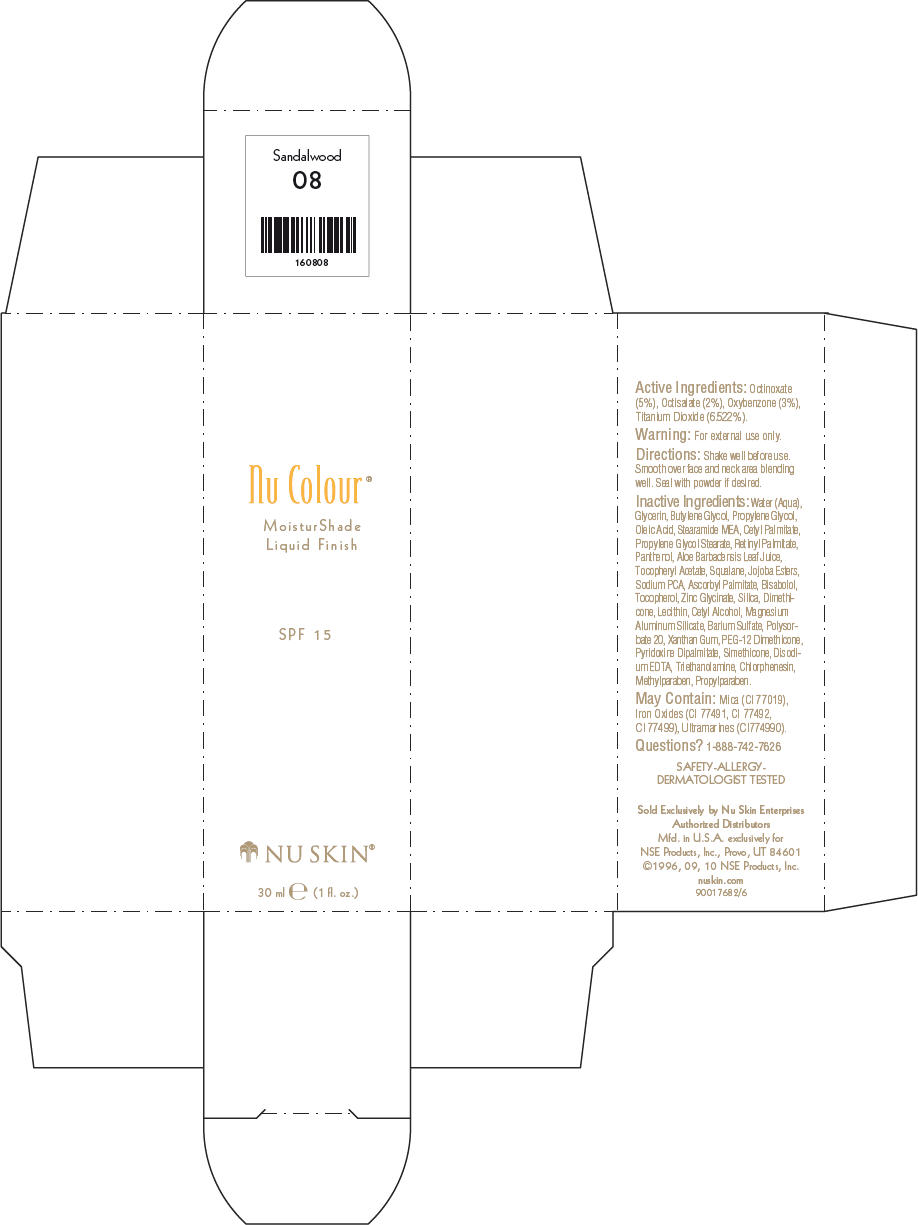
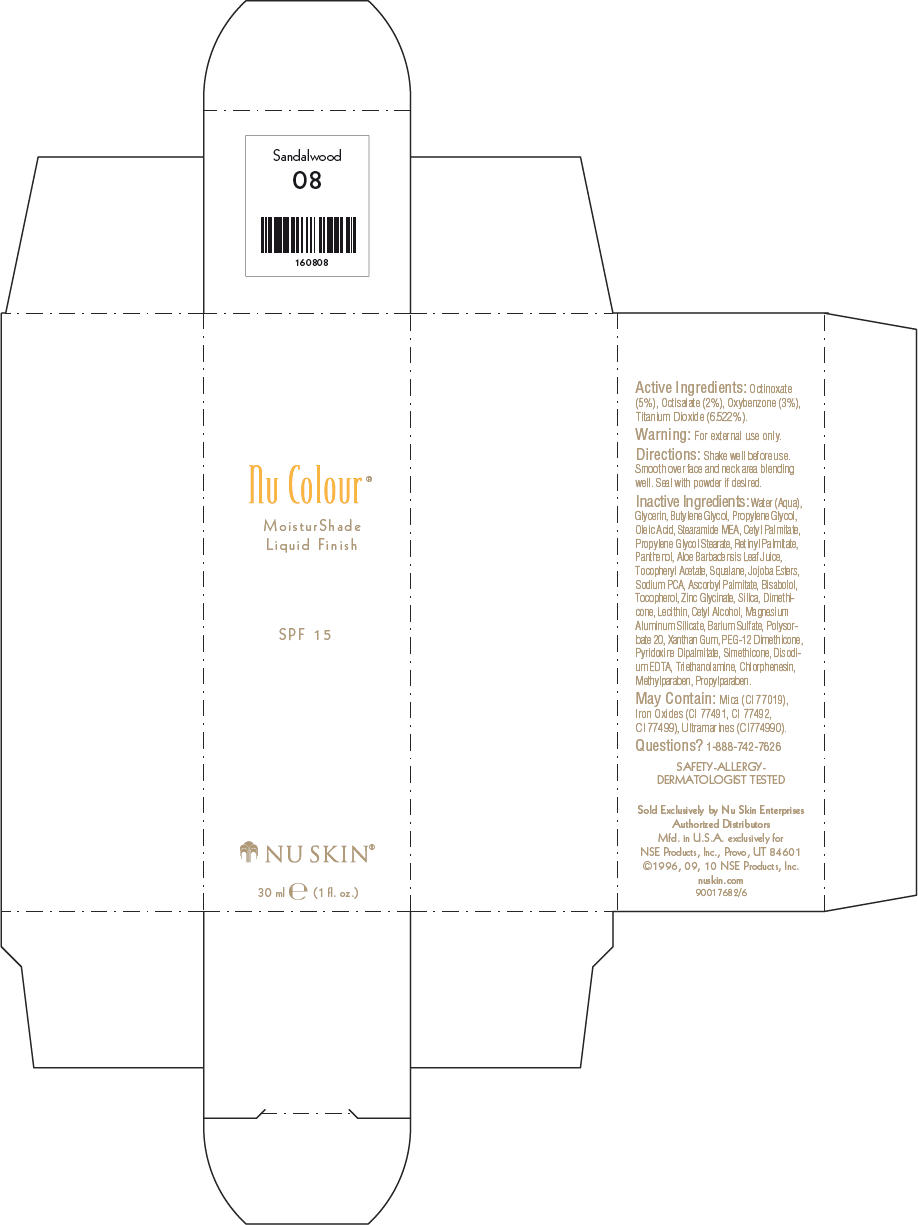
- PRINCIPAL DISPLAY PANEL - 30 ml Sandalwood Carton
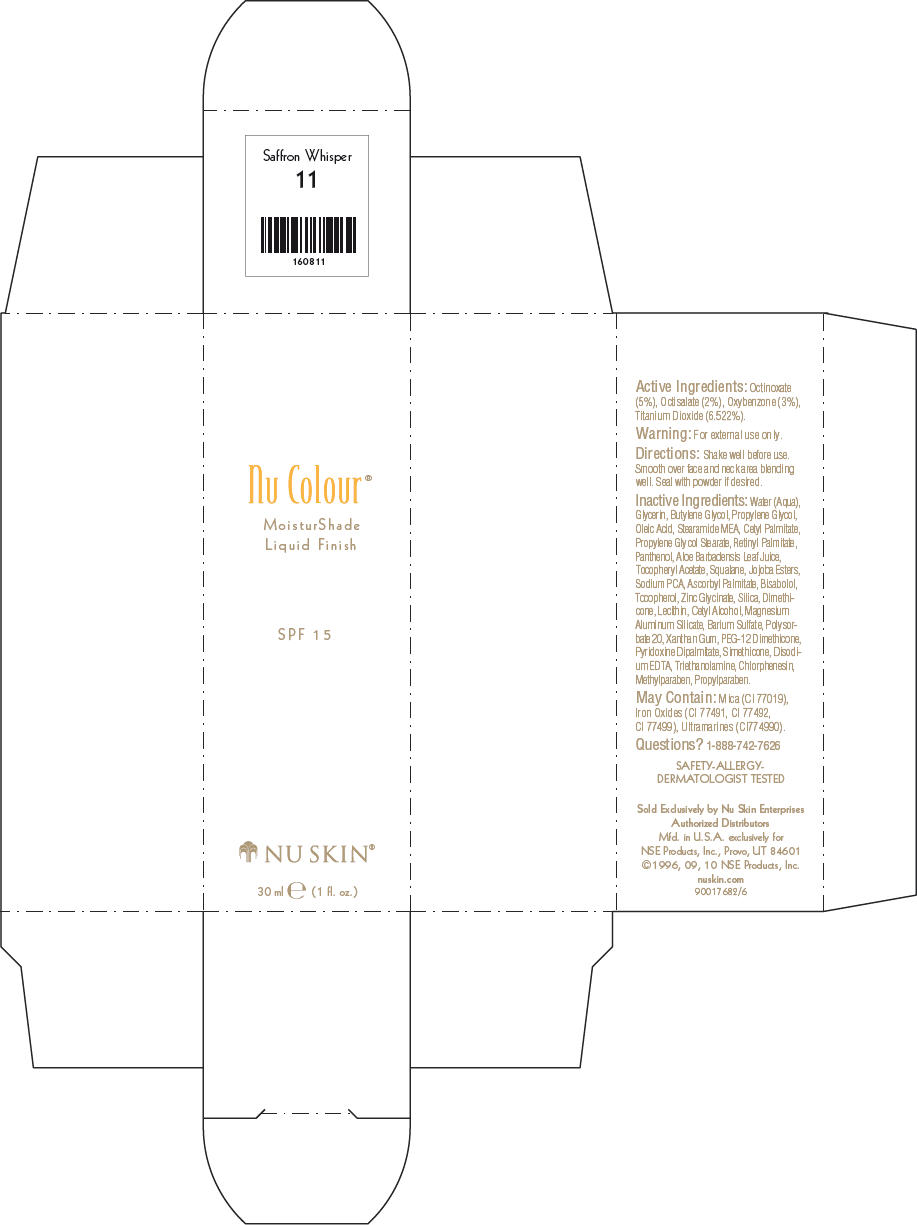
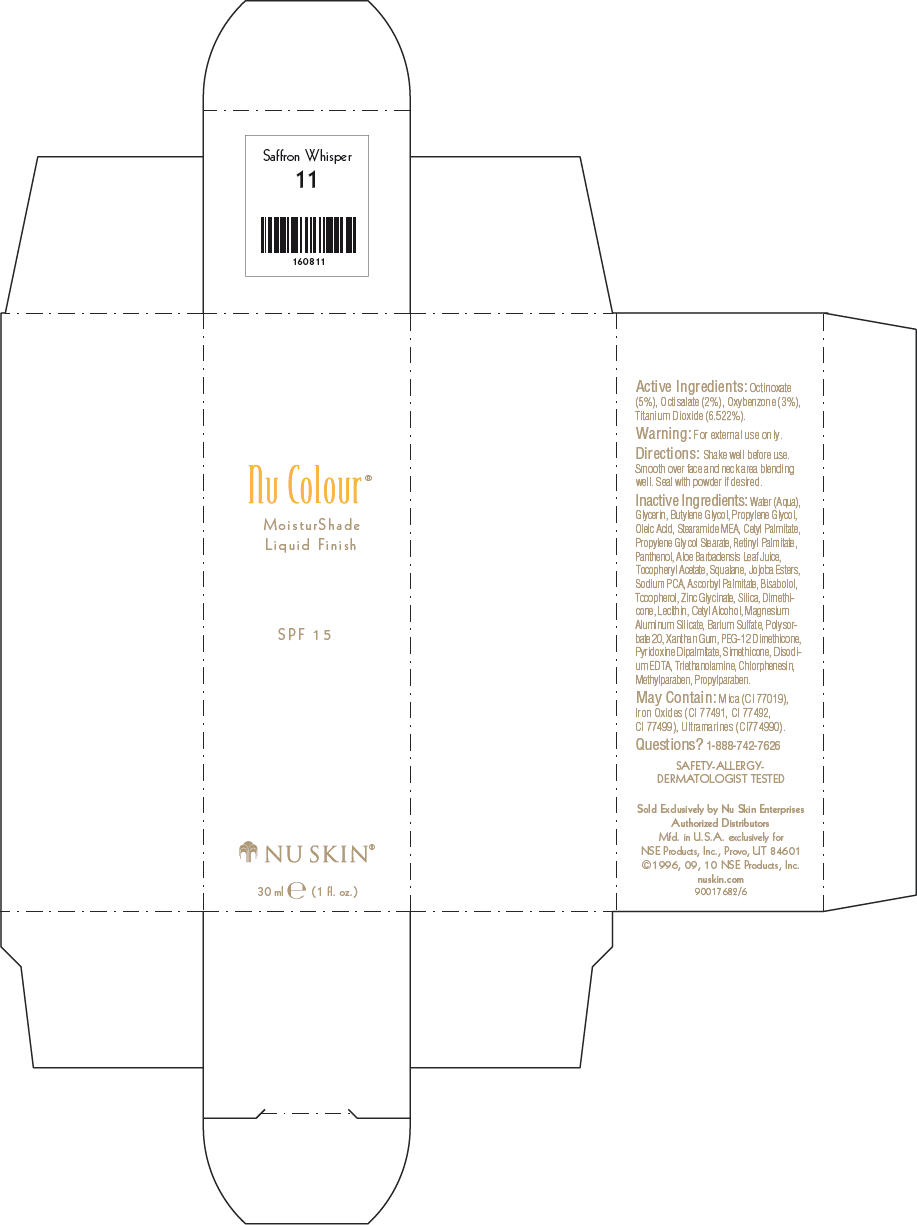
- PRINCIPAL DISPLAY PANEL - 30 ml Saffron Whisper Carton
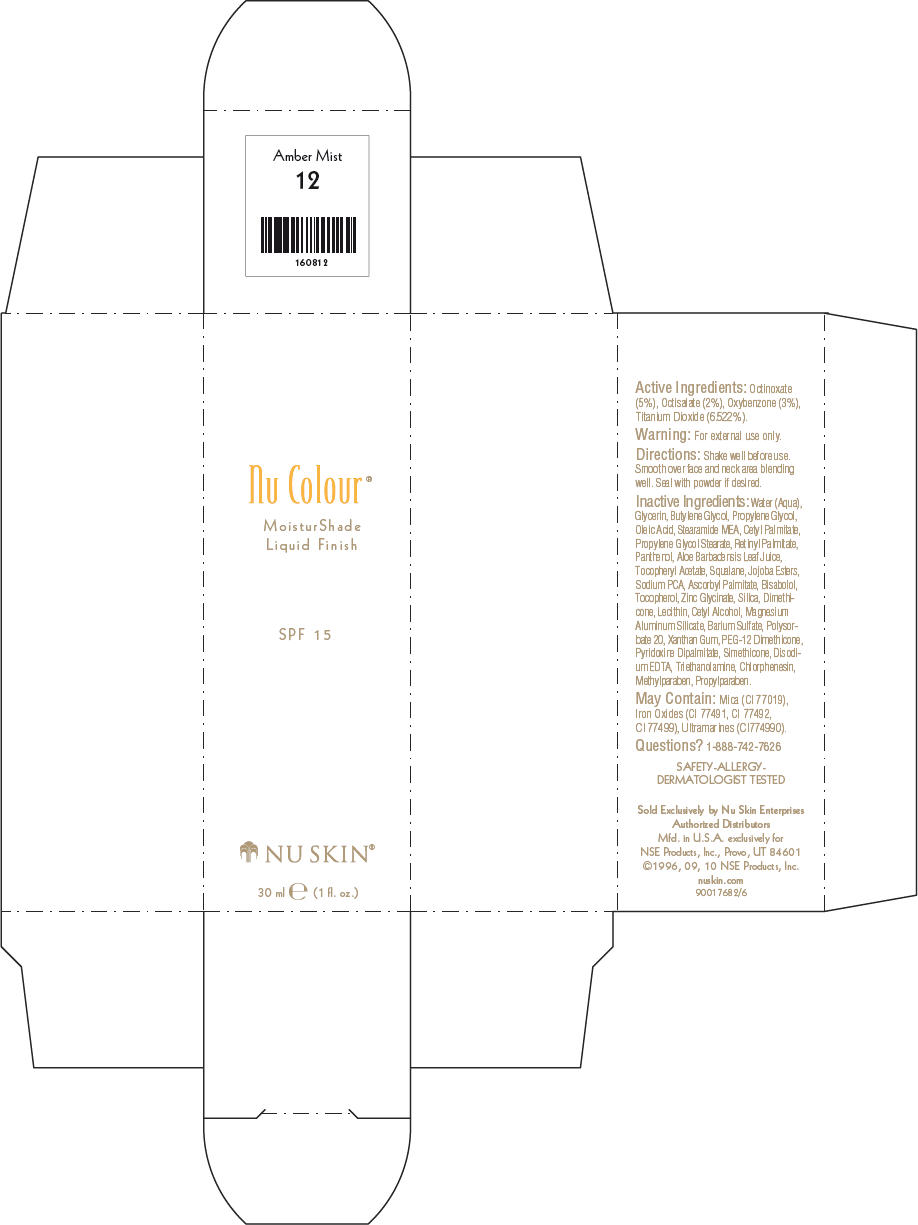
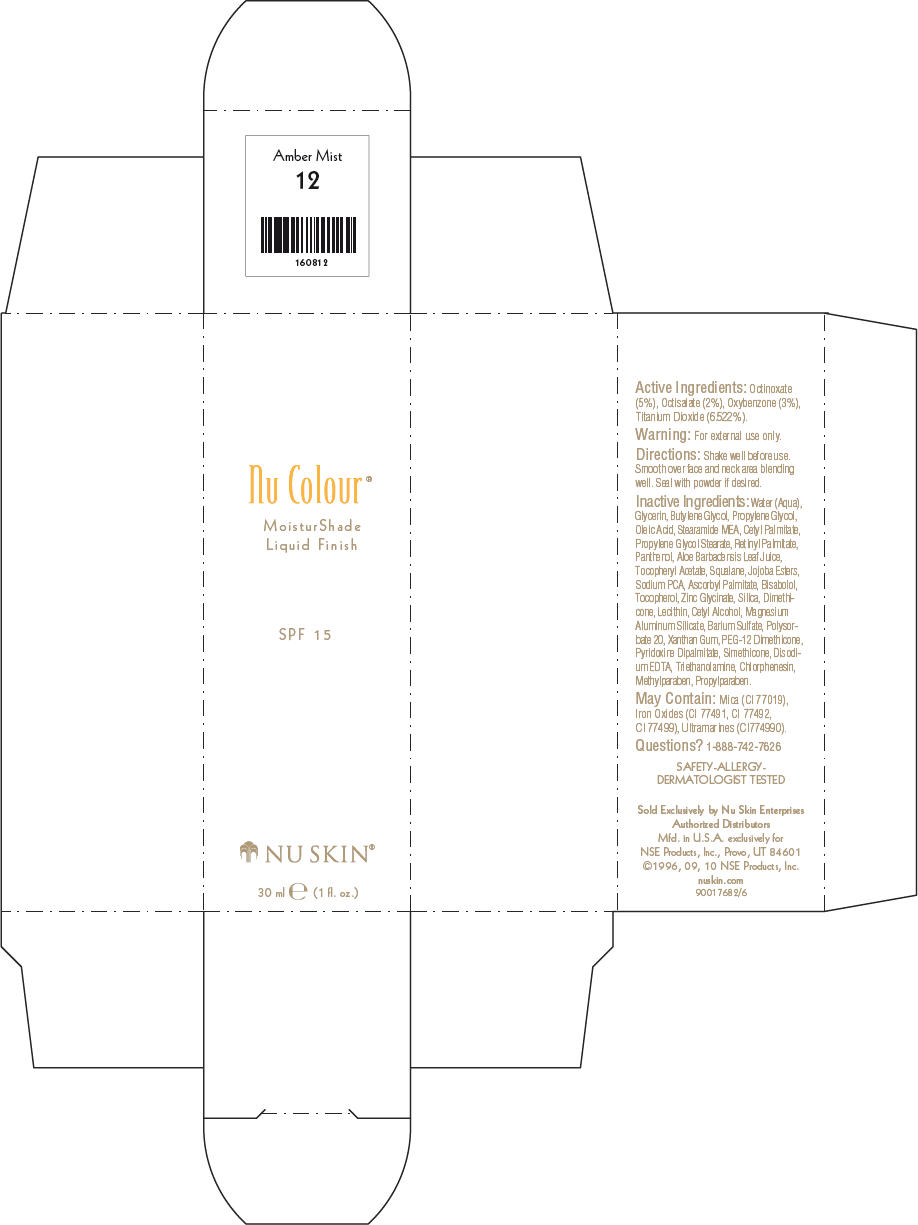
- PRINCIPAL DISPLAY PANEL - 30 ml Amber Mist Carton
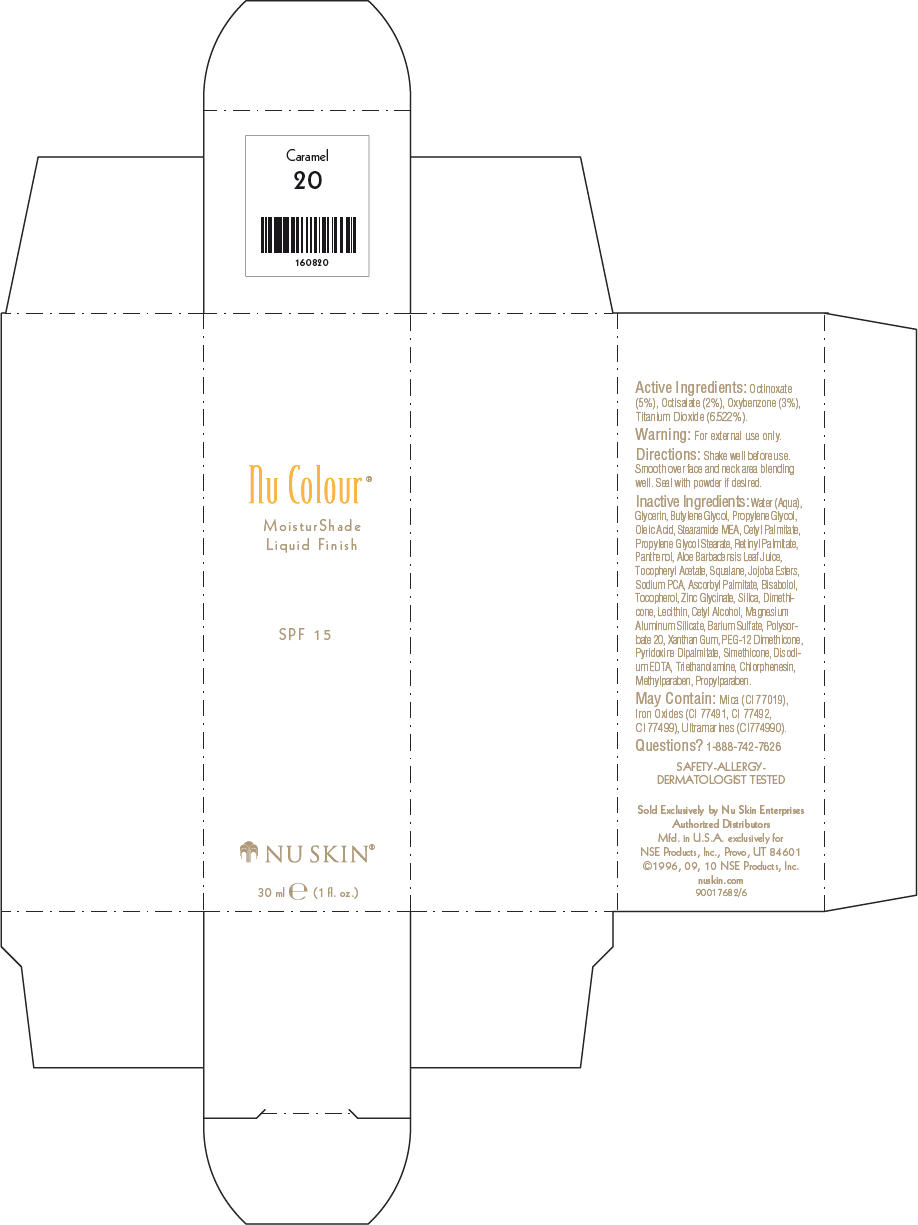
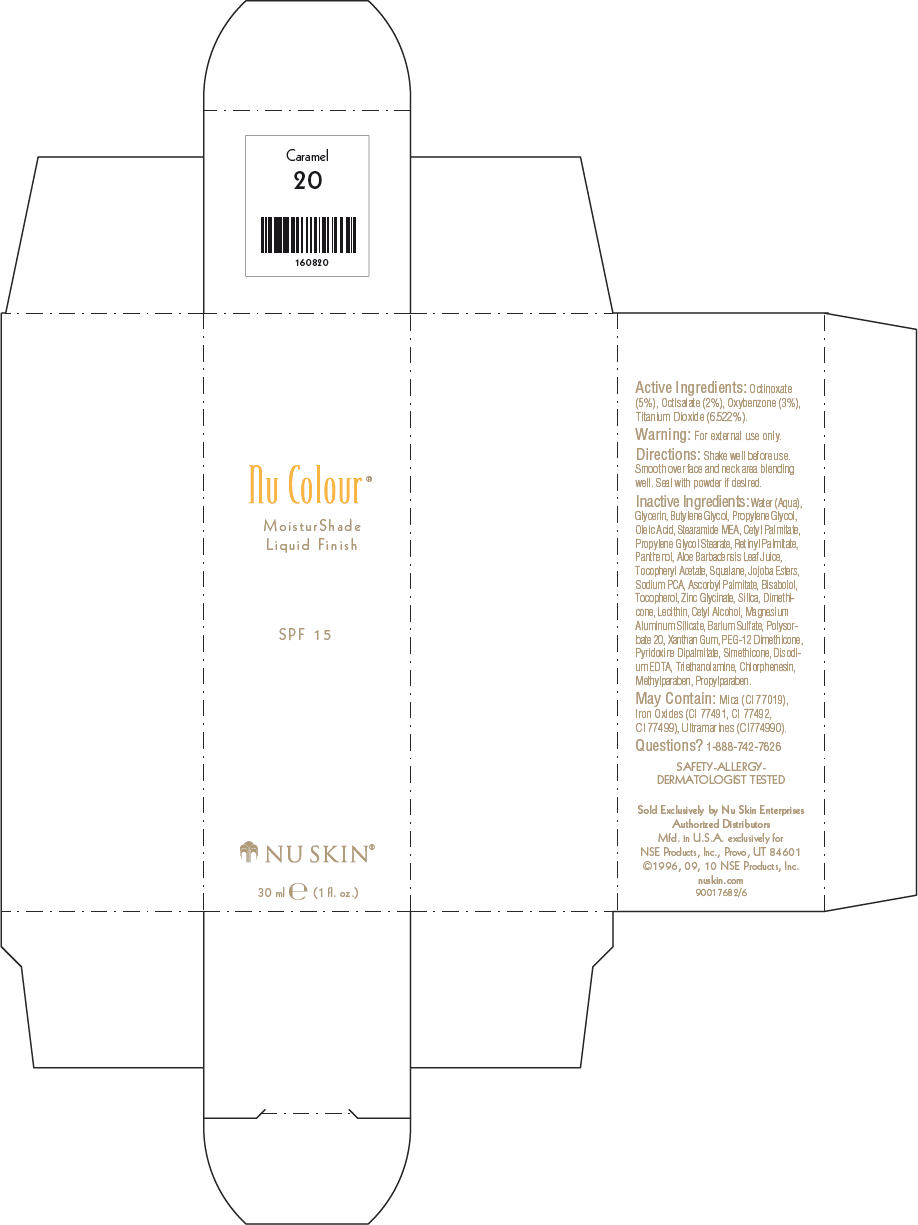
- PRINCIPAL DISPLAY PANEL - 30 ml Caramel Carton
-
INGREDIENTS AND APPEARANCE
NU SKIN NU COLOUR MOISTURSHADE FINISH SPF 15 DELICATE IVORY
octinoxate, octisalate, oxybenzone, and titanium dioxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62839-0802 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 50 g in 1000 mL Octisalate (UNII: 4X49Y0596W) (Octisalate - UNII:4X49Y0596W) Octisalate 20 g in 1000 mL Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 30 g in 1000 mL Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 65.22 g in 1000 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Glycerin (UNII: PDC6A3C0OX) Butylene Glycol (UNII: 3XUS85K0RA) Propylene Glycol (UNII: 6DC9Q167V3) Oleic Acid (UNII: 2UMI9U37CP) Stearic Monoethanolamide (UNII: 03XV449Q24) Cetyl Palmitate (UNII: 5ZA2S6B08X) Propylene Glycol Monostearate (UNII: F76354LMGR) Vitamin A Palmitate (UNII: 1D1K0N0VVC) Panthenol (UNII: WV9CM0O67Z) Aloe Vera Leaf (UNII: ZY81Z83H0X) Squalene (UNII: 7QWM220FJH) Sodium Pyrrolidone Carboxylate (UNII: 469OTG57A2) Ascorbyl Palmitate (UNII: QN83US2B0N) Levomenol (UNII: 24WE03BX2T) Tocopherol (UNII: R0ZB2556P8) Silicon Dioxide (UNII: ETJ7Z6XBU4) Dimethicone (UNII: 92RU3N3Y1O) Cetyl Alcohol (UNII: 936JST6JCN) Magnesium Aluminum Silicate (UNII: 6M3P64V0NC) Barium Sulfate (UNII: 25BB7EKE2E) Polysorbate 20 (UNII: 7T1F30V5YH) Xanthan Gum (UNII: TTV12P4NEE) PEG-12 Dimethicone (UNII: ZEL54N6W95) Edetate Disodium (UNII: 7FLD91C86K) Trolamine (UNII: 9O3K93S3TK) Chlorphenesin (UNII: I670DAL4SZ) Methylparaben (UNII: A2I8C7HI9T) Propylparaben (UNII: Z8IX2SC1OH) Mica (UNII: V8A1AW0880) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62839-0802-1 1 in 1 CARTON 1 30 mL in 1 BOTTLE, PUMP Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 01/03/2011 NU SKIN NU COLOUR MOISTURSHADE FINISH SPF 15 SHELL ROSE
octinoxate, octisalate, oxybenzone, and titanium dioxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62839-0803 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 50 g in 1000 mL Octisalate (UNII: 4X49Y0596W) (Octisalate - UNII:4X49Y0596W) Octisalate 20 g in 1000 mL Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 30 g in 1000 mL Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 65.22 g in 1000 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Glycerin (UNII: PDC6A3C0OX) Butylene Glycol (UNII: 3XUS85K0RA) Propylene Glycol (UNII: 6DC9Q167V3) Oleic Acid (UNII: 2UMI9U37CP) Stearic Monoethanolamide (UNII: 03XV449Q24) Cetyl Palmitate (UNII: 5ZA2S6B08X) Propylene Glycol Monostearate (UNII: F76354LMGR) Vitamin A Palmitate (UNII: 1D1K0N0VVC) Panthenol (UNII: WV9CM0O67Z) Aloe Vera Leaf (UNII: ZY81Z83H0X) Squalene (UNII: 7QWM220FJH) Sodium Pyrrolidone Carboxylate (UNII: 469OTG57A2) Ascorbyl Palmitate (UNII: QN83US2B0N) Levomenol (UNII: 24WE03BX2T) Tocopherol (UNII: R0ZB2556P8) Silicon Dioxide (UNII: ETJ7Z6XBU4) Dimethicone (UNII: 92RU3N3Y1O) Cetyl Alcohol (UNII: 936JST6JCN) Magnesium Aluminum Silicate (UNII: 6M3P64V0NC) Barium Sulfate (UNII: 25BB7EKE2E) Polysorbate 20 (UNII: 7T1F30V5YH) Xanthan Gum (UNII: TTV12P4NEE) PEG-12 Dimethicone (UNII: ZEL54N6W95) Edetate Disodium (UNII: 7FLD91C86K) Trolamine (UNII: 9O3K93S3TK) Chlorphenesin (UNII: I670DAL4SZ) Methylparaben (UNII: A2I8C7HI9T) Propylparaben (UNII: Z8IX2SC1OH) Mica (UNII: V8A1AW0880) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62839-0803-1 1 in 1 CARTON 1 30 mL in 1 BOTTLE, PUMP Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 01/03/2011 NU SKIN NU COLOUR MOISTURSHADE FINISH SPF 15 NATURAL BEIGE
octinoxate, octisalate, oxybenzone, and titanium dioxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62839-0804 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 50 g in 1000 mL Octisalate (UNII: 4X49Y0596W) (Octisalate - UNII:4X49Y0596W) Octisalate 20 g in 1000 mL Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 30 g in 1000 mL Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 65.22 g in 1000 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Glycerin (UNII: PDC6A3C0OX) Butylene Glycol (UNII: 3XUS85K0RA) Propylene Glycol (UNII: 6DC9Q167V3) Oleic Acid (UNII: 2UMI9U37CP) Stearic Monoethanolamide (UNII: 03XV449Q24) Cetyl Palmitate (UNII: 5ZA2S6B08X) Propylene Glycol Monostearate (UNII: F76354LMGR) Vitamin A Palmitate (UNII: 1D1K0N0VVC) Panthenol (UNII: WV9CM0O67Z) Aloe Vera Leaf (UNII: ZY81Z83H0X) Squalene (UNII: 7QWM220FJH) Sodium Pyrrolidone Carboxylate (UNII: 469OTG57A2) Ascorbyl Palmitate (UNII: QN83US2B0N) Levomenol (UNII: 24WE03BX2T) Tocopherol (UNII: R0ZB2556P8) Silicon Dioxide (UNII: ETJ7Z6XBU4) Dimethicone (UNII: 92RU3N3Y1O) Cetyl Alcohol (UNII: 936JST6JCN) Magnesium Aluminum Silicate (UNII: 6M3P64V0NC) Barium Sulfate (UNII: 25BB7EKE2E) Polysorbate 20 (UNII: 7T1F30V5YH) Xanthan Gum (UNII: TTV12P4NEE) PEG-12 Dimethicone (UNII: ZEL54N6W95) Edetate Disodium (UNII: 7FLD91C86K) Trolamine (UNII: 9O3K93S3TK) Chlorphenesin (UNII: I670DAL4SZ) Methylparaben (UNII: A2I8C7HI9T) Propylparaben (UNII: Z8IX2SC1OH) Mica (UNII: V8A1AW0880) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62839-0804-1 1 in 1 CARTON 1 30 mL in 1 BOTTLE, PUMP Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 01/03/2011 NU SKIN NU COLOUR MOISTURSHADE FINISH SPF 15 HONEY BEIGE
octinoxate, octisalate, oxybenzone, and titanium dioxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62839-0805 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 50 g in 1000 mL Octisalate (UNII: 4X49Y0596W) (Octisalate - UNII:4X49Y0596W) Octisalate 20 g in 1000 mL Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 30 g in 1000 mL Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 65.22 g in 1000 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Glycerin (UNII: PDC6A3C0OX) Butylene Glycol (UNII: 3XUS85K0RA) Propylene Glycol (UNII: 6DC9Q167V3) Oleic Acid (UNII: 2UMI9U37CP) Stearic Monoethanolamide (UNII: 03XV449Q24) Cetyl Palmitate (UNII: 5ZA2S6B08X) Propylene Glycol Monostearate (UNII: F76354LMGR) Vitamin A Palmitate (UNII: 1D1K0N0VVC) Panthenol (UNII: WV9CM0O67Z) Aloe Vera Leaf (UNII: ZY81Z83H0X) Squalene (UNII: 7QWM220FJH) Sodium Pyrrolidone Carboxylate (UNII: 469OTG57A2) Ascorbyl Palmitate (UNII: QN83US2B0N) Levomenol (UNII: 24WE03BX2T) Tocopherol (UNII: R0ZB2556P8) Silicon Dioxide (UNII: ETJ7Z6XBU4) Dimethicone (UNII: 92RU3N3Y1O) Cetyl Alcohol (UNII: 936JST6JCN) Magnesium Aluminum Silicate (UNII: 6M3P64V0NC) Barium Sulfate (UNII: 25BB7EKE2E) Polysorbate 20 (UNII: 7T1F30V5YH) Xanthan Gum (UNII: TTV12P4NEE) PEG-12 Dimethicone (UNII: ZEL54N6W95) Edetate Disodium (UNII: 7FLD91C86K) Trolamine (UNII: 9O3K93S3TK) Chlorphenesin (UNII: I670DAL4SZ) Methylparaben (UNII: A2I8C7HI9T) Propylparaben (UNII: Z8IX2SC1OH) Mica (UNII: V8A1AW0880) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62839-0805-1 1 in 1 CARTON 1 30 mL in 1 BOTTLE, PUMP Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 01/03/2011 NU SKIN NU COLOUR MOISTURSHADE FINISH SPF 15 GOLDEN BEIGE
octinoxate, octisalate, oxybenzone, and titanium dioxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62839-0806 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 50 g in 1000 mL Octisalate (UNII: 4X49Y0596W) (Octisalate - UNII:4X49Y0596W) Octisalate 20 g in 1000 mL Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 30 g in 1000 mL Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 65.22 g in 1000 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Glycerin (UNII: PDC6A3C0OX) Butylene Glycol (UNII: 3XUS85K0RA) Propylene Glycol (UNII: 6DC9Q167V3) Oleic Acid (UNII: 2UMI9U37CP) Stearic Monoethanolamide (UNII: 03XV449Q24) Cetyl Palmitate (UNII: 5ZA2S6B08X) Propylene Glycol Monostearate (UNII: F76354LMGR) Vitamin A Palmitate (UNII: 1D1K0N0VVC) Panthenol (UNII: WV9CM0O67Z) Aloe Vera Leaf (UNII: ZY81Z83H0X) Squalene (UNII: 7QWM220FJH) Sodium Pyrrolidone Carboxylate (UNII: 469OTG57A2) Ascorbyl Palmitate (UNII: QN83US2B0N) Levomenol (UNII: 24WE03BX2T) Tocopherol (UNII: R0ZB2556P8) Silicon Dioxide (UNII: ETJ7Z6XBU4) Dimethicone (UNII: 92RU3N3Y1O) Cetyl Alcohol (UNII: 936JST6JCN) Magnesium Aluminum Silicate (UNII: 6M3P64V0NC) Barium Sulfate (UNII: 25BB7EKE2E) Polysorbate 20 (UNII: 7T1F30V5YH) Xanthan Gum (UNII: TTV12P4NEE) PEG-12 Dimethicone (UNII: ZEL54N6W95) Edetate Disodium (UNII: 7FLD91C86K) Trolamine (UNII: 9O3K93S3TK) Chlorphenesin (UNII: I670DAL4SZ) Methylparaben (UNII: A2I8C7HI9T) Propylparaben (UNII: Z8IX2SC1OH) Mica (UNII: V8A1AW0880) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62839-0806-1 1 in 1 CARTON 1 30 mL in 1 BOTTLE, PUMP Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 01/03/2011 NU SKIN NU COLOUR MOISTURSHADE FINISH SPF 15 SUNSET BEIGE
octinoxate, octisalate, oxybenzone, and titanium dioxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62839-0807 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 50 g in 1000 mL Octisalate (UNII: 4X49Y0596W) (Octisalate - UNII:4X49Y0596W) Octisalate 20 g in 1000 mL Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 30 g in 1000 mL Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 65.22 g in 1000 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Glycerin (UNII: PDC6A3C0OX) Butylene Glycol (UNII: 3XUS85K0RA) Propylene Glycol (UNII: 6DC9Q167V3) Oleic Acid (UNII: 2UMI9U37CP) Stearic Monoethanolamide (UNII: 03XV449Q24) Cetyl Palmitate (UNII: 5ZA2S6B08X) Propylene Glycol Monostearate (UNII: F76354LMGR) Vitamin A Palmitate (UNII: 1D1K0N0VVC) Panthenol (UNII: WV9CM0O67Z) Aloe Vera Leaf (UNII: ZY81Z83H0X) Squalene (UNII: 7QWM220FJH) Sodium Pyrrolidone Carboxylate (UNII: 469OTG57A2) Ascorbyl Palmitate (UNII: QN83US2B0N) Levomenol (UNII: 24WE03BX2T) Tocopherol (UNII: R0ZB2556P8) Silicon Dioxide (UNII: ETJ7Z6XBU4) Dimethicone (UNII: 92RU3N3Y1O) Cetyl Alcohol (UNII: 936JST6JCN) Magnesium Aluminum Silicate (UNII: 6M3P64V0NC) Barium Sulfate (UNII: 25BB7EKE2E) Polysorbate 20 (UNII: 7T1F30V5YH) Xanthan Gum (UNII: TTV12P4NEE) PEG-12 Dimethicone (UNII: ZEL54N6W95) Edetate Disodium (UNII: 7FLD91C86K) Trolamine (UNII: 9O3K93S3TK) Chlorphenesin (UNII: I670DAL4SZ) Methylparaben (UNII: A2I8C7HI9T) Propylparaben (UNII: Z8IX2SC1OH) Mica (UNII: V8A1AW0880) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62839-0807-1 1 in 1 CARTON 1 30 mL in 1 BOTTLE, PUMP Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 01/03/2011 NU SKIN NU COLOUR MOISTURSHADE FINISH SPF 15 SANDALWOOD
octinoxate, octisalate, oxybenzone, and titanium dioxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62839-0808 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 50 g in 1000 mL Octisalate (UNII: 4X49Y0596W) (Octisalate - UNII:4X49Y0596W) Octisalate 20 g in 1000 mL Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 30 g in 1000 mL Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 65.22 g in 1000 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Glycerin (UNII: PDC6A3C0OX) Butylene Glycol (UNII: 3XUS85K0RA) Propylene Glycol (UNII: 6DC9Q167V3) Oleic Acid (UNII: 2UMI9U37CP) Stearic Monoethanolamide (UNII: 03XV449Q24) Cetyl Palmitate (UNII: 5ZA2S6B08X) Propylene Glycol Monostearate (UNII: F76354LMGR) Vitamin A Palmitate (UNII: 1D1K0N0VVC) Panthenol (UNII: WV9CM0O67Z) Aloe Vera Leaf (UNII: ZY81Z83H0X) Squalene (UNII: 7QWM220FJH) Sodium Pyrrolidone Carboxylate (UNII: 469OTG57A2) Ascorbyl Palmitate (UNII: QN83US2B0N) Levomenol (UNII: 24WE03BX2T) Tocopherol (UNII: R0ZB2556P8) Silicon Dioxide (UNII: ETJ7Z6XBU4) Dimethicone (UNII: 92RU3N3Y1O) Cetyl Alcohol (UNII: 936JST6JCN) Magnesium Aluminum Silicate (UNII: 6M3P64V0NC) Barium Sulfate (UNII: 25BB7EKE2E) Polysorbate 20 (UNII: 7T1F30V5YH) Xanthan Gum (UNII: TTV12P4NEE) PEG-12 Dimethicone (UNII: ZEL54N6W95) Edetate Disodium (UNII: 7FLD91C86K) Trolamine (UNII: 9O3K93S3TK) Chlorphenesin (UNII: I670DAL4SZ) Methylparaben (UNII: A2I8C7HI9T) Propylparaben (UNII: Z8IX2SC1OH) Mica (UNII: V8A1AW0880) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62839-0808-1 1 in 1 CARTON 1 30 mL in 1 BOTTLE, PUMP Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 01/03/2011 NU SKIN NU COLOUR MOISTURSHADE FINISH SPF 15 SAFFRON WHISPER
octinoxate, octisalate, oxybenzone, and titanium dioxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62839-0811 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 50 g in 1000 mL Octisalate (UNII: 4X49Y0596W) (Octisalate - UNII:4X49Y0596W) Octisalate 20 g in 1000 mL Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 30 g in 1000 mL Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 65.22 g in 1000 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Glycerin (UNII: PDC6A3C0OX) Butylene Glycol (UNII: 3XUS85K0RA) Propylene Glycol (UNII: 6DC9Q167V3) Oleic Acid (UNII: 2UMI9U37CP) Stearic Monoethanolamide (UNII: 03XV449Q24) Cetyl Palmitate (UNII: 5ZA2S6B08X) Propylene Glycol Monostearate (UNII: F76354LMGR) Vitamin A Palmitate (UNII: 1D1K0N0VVC) Panthenol (UNII: WV9CM0O67Z) Aloe Vera Leaf (UNII: ZY81Z83H0X) Squalene (UNII: 7QWM220FJH) Sodium Pyrrolidone Carboxylate (UNII: 469OTG57A2) Ascorbyl Palmitate (UNII: QN83US2B0N) Levomenol (UNII: 24WE03BX2T) Tocopherol (UNII: R0ZB2556P8) Silicon Dioxide (UNII: ETJ7Z6XBU4) Dimethicone (UNII: 92RU3N3Y1O) Cetyl Alcohol (UNII: 936JST6JCN) Magnesium Aluminum Silicate (UNII: 6M3P64V0NC) Barium Sulfate (UNII: 25BB7EKE2E) Polysorbate 20 (UNII: 7T1F30V5YH) Xanthan Gum (UNII: TTV12P4NEE) PEG-12 Dimethicone (UNII: ZEL54N6W95) Edetate Disodium (UNII: 7FLD91C86K) Trolamine (UNII: 9O3K93S3TK) Chlorphenesin (UNII: I670DAL4SZ) Methylparaben (UNII: A2I8C7HI9T) Propylparaben (UNII: Z8IX2SC1OH) Mica (UNII: V8A1AW0880) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62839-0811-1 1 in 1 CARTON 1 30 mL in 1 BOTTLE, PUMP Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 01/03/2011 NU SKIN NU COLOUR MOISTURSHADE FINISH SPF 15 AMBER MIST
octinoxate, octisalate, oxybenzone, and titanium dioxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62839-0812 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 50 g in 1000 mL Octisalate (UNII: 4X49Y0596W) (Octisalate - UNII:4X49Y0596W) Octisalate 20 g in 1000 mL Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 30 g in 1000 mL Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 65.22 g in 1000 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Glycerin (UNII: PDC6A3C0OX) Butylene Glycol (UNII: 3XUS85K0RA) Propylene Glycol (UNII: 6DC9Q167V3) Oleic Acid (UNII: 2UMI9U37CP) Stearic Monoethanolamide (UNII: 03XV449Q24) Cetyl Palmitate (UNII: 5ZA2S6B08X) Propylene Glycol Monostearate (UNII: F76354LMGR) Vitamin A Palmitate (UNII: 1D1K0N0VVC) Panthenol (UNII: WV9CM0O67Z) Aloe Vera Leaf (UNII: ZY81Z83H0X) Squalene (UNII: 7QWM220FJH) Sodium Pyrrolidone Carboxylate (UNII: 469OTG57A2) Ascorbyl Palmitate (UNII: QN83US2B0N) Levomenol (UNII: 24WE03BX2T) Tocopherol (UNII: R0ZB2556P8) Silicon Dioxide (UNII: ETJ7Z6XBU4) Dimethicone (UNII: 92RU3N3Y1O) Cetyl Alcohol (UNII: 936JST6JCN) Magnesium Aluminum Silicate (UNII: 6M3P64V0NC) Barium Sulfate (UNII: 25BB7EKE2E) Polysorbate 20 (UNII: 7T1F30V5YH) Xanthan Gum (UNII: TTV12P4NEE) PEG-12 Dimethicone (UNII: ZEL54N6W95) Edetate Disodium (UNII: 7FLD91C86K) Trolamine (UNII: 9O3K93S3TK) Chlorphenesin (UNII: I670DAL4SZ) Methylparaben (UNII: A2I8C7HI9T) Propylparaben (UNII: Z8IX2SC1OH) Mica (UNII: V8A1AW0880) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62839-0812-1 1 in 1 CARTON 1 30 mL in 1 BOTTLE, PUMP Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 01/03/2011 NU SKIN NU COLOUR MOISTURSHADE FINISH SPF 15 CARAMEL
octinoxate, octisalate, oxybenzone, and titanium dioxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62839-0820 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 50 g in 1000 mL Octisalate (UNII: 4X49Y0596W) (Octisalate - UNII:4X49Y0596W) Octisalate 20 g in 1000 mL Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 30 g in 1000 mL Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 65.22 g in 1000 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Glycerin (UNII: PDC6A3C0OX) Butylene Glycol (UNII: 3XUS85K0RA) Propylene Glycol (UNII: 6DC9Q167V3) Oleic Acid (UNII: 2UMI9U37CP) Stearic Monoethanolamide (UNII: 03XV449Q24) Cetyl Palmitate (UNII: 5ZA2S6B08X) Propylene Glycol Monostearate (UNII: F76354LMGR) Vitamin A Palmitate (UNII: 1D1K0N0VVC) Panthenol (UNII: WV9CM0O67Z) Aloe Vera Leaf (UNII: ZY81Z83H0X) Squalene (UNII: 7QWM220FJH) Sodium Pyrrolidone Carboxylate (UNII: 469OTG57A2) Ascorbyl Palmitate (UNII: QN83US2B0N) Levomenol (UNII: 24WE03BX2T) Tocopherol (UNII: R0ZB2556P8) Silicon Dioxide (UNII: ETJ7Z6XBU4) Dimethicone (UNII: 92RU3N3Y1O) Cetyl Alcohol (UNII: 936JST6JCN) Magnesium Aluminum Silicate (UNII: 6M3P64V0NC) Barium Sulfate (UNII: 25BB7EKE2E) Polysorbate 20 (UNII: 7T1F30V5YH) Xanthan Gum (UNII: TTV12P4NEE) PEG-12 Dimethicone (UNII: ZEL54N6W95) Edetate Disodium (UNII: 7FLD91C86K) Trolamine (UNII: 9O3K93S3TK) Chlorphenesin (UNII: I670DAL4SZ) Methylparaben (UNII: A2I8C7HI9T) Propylparaben (UNII: Z8IX2SC1OH) Mica (UNII: V8A1AW0880) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62839-0820-1 1 in 1 CARTON 1 30 mL in 1 BOTTLE, PUMP Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 01/03/2011 Labeler - NSE Products, Inc. (966817975)