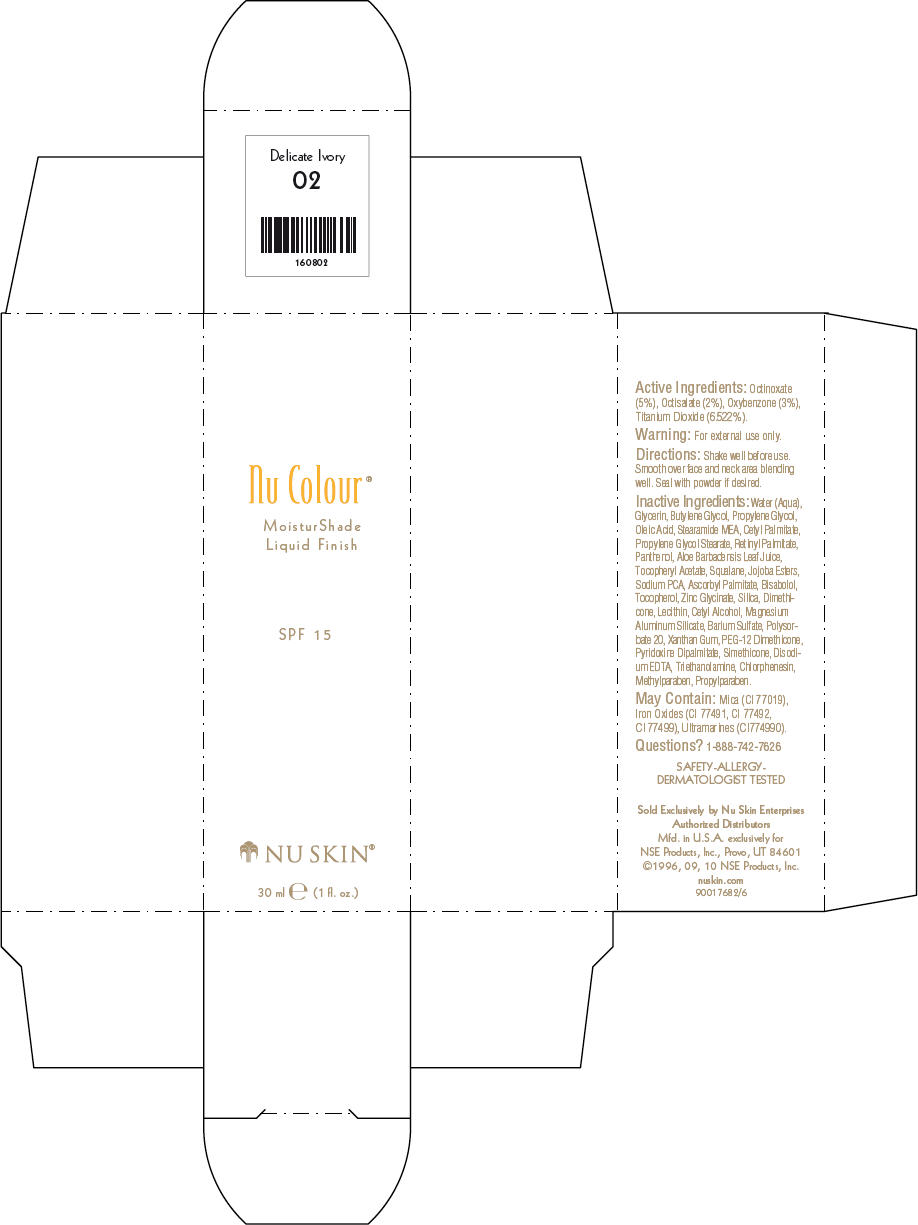
NU SKIN NU COLOUR MOISTURSHADE FINISH SPF 15 DELICATE IVORY- octinoxate, octisalate, oxybenzone, and titanium dioxide lotion
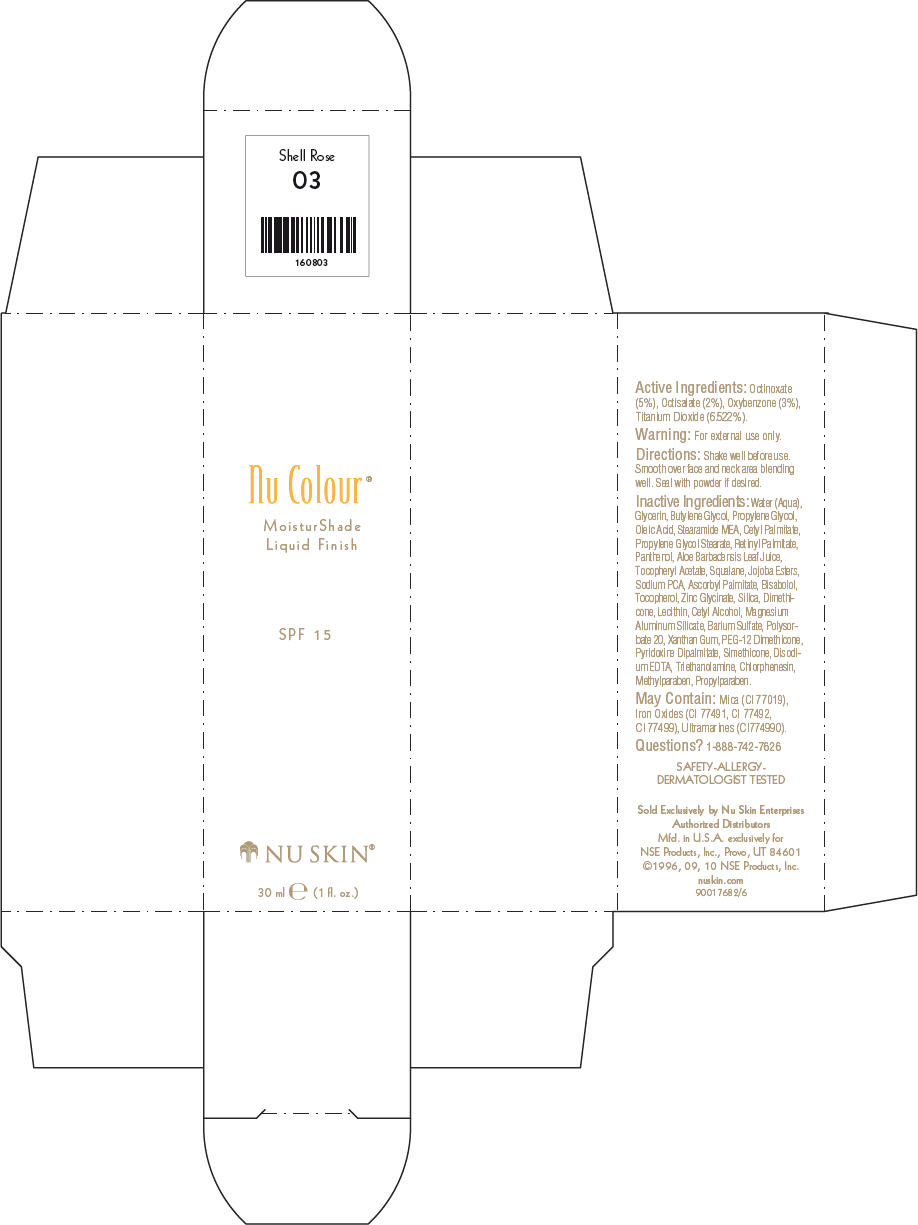
NU SKIN NU COLOUR MOISTURSHADE FINISH SPF 15 SHELL ROSE- octinoxate, octisalate, oxybenzone, and titanium dioxide lotion
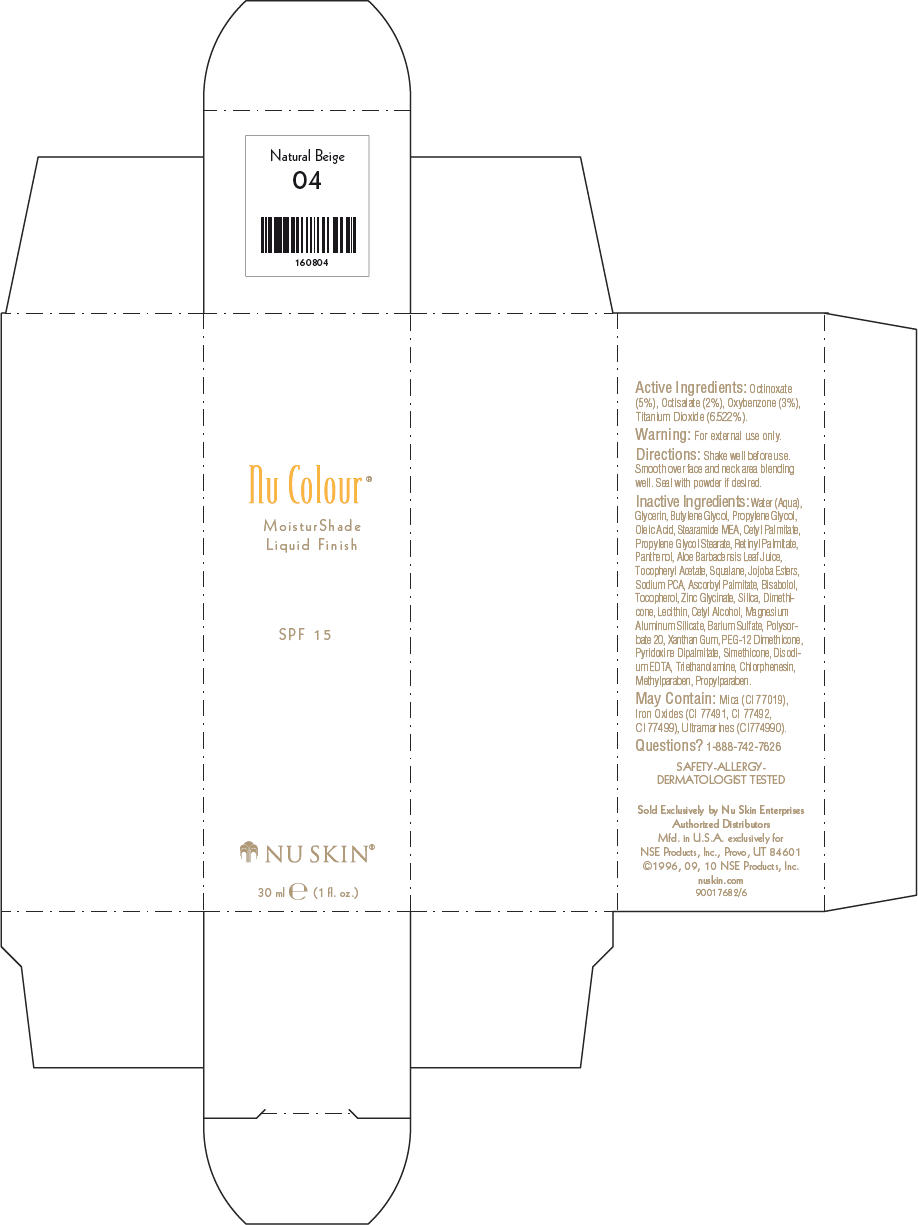
NU SKIN NU COLOUR MOISTURSHADE FINISH SPF 15 NATURAL BEIGE- octinoxate, octisalate, oxybenzone, and titanium dioxide lotion
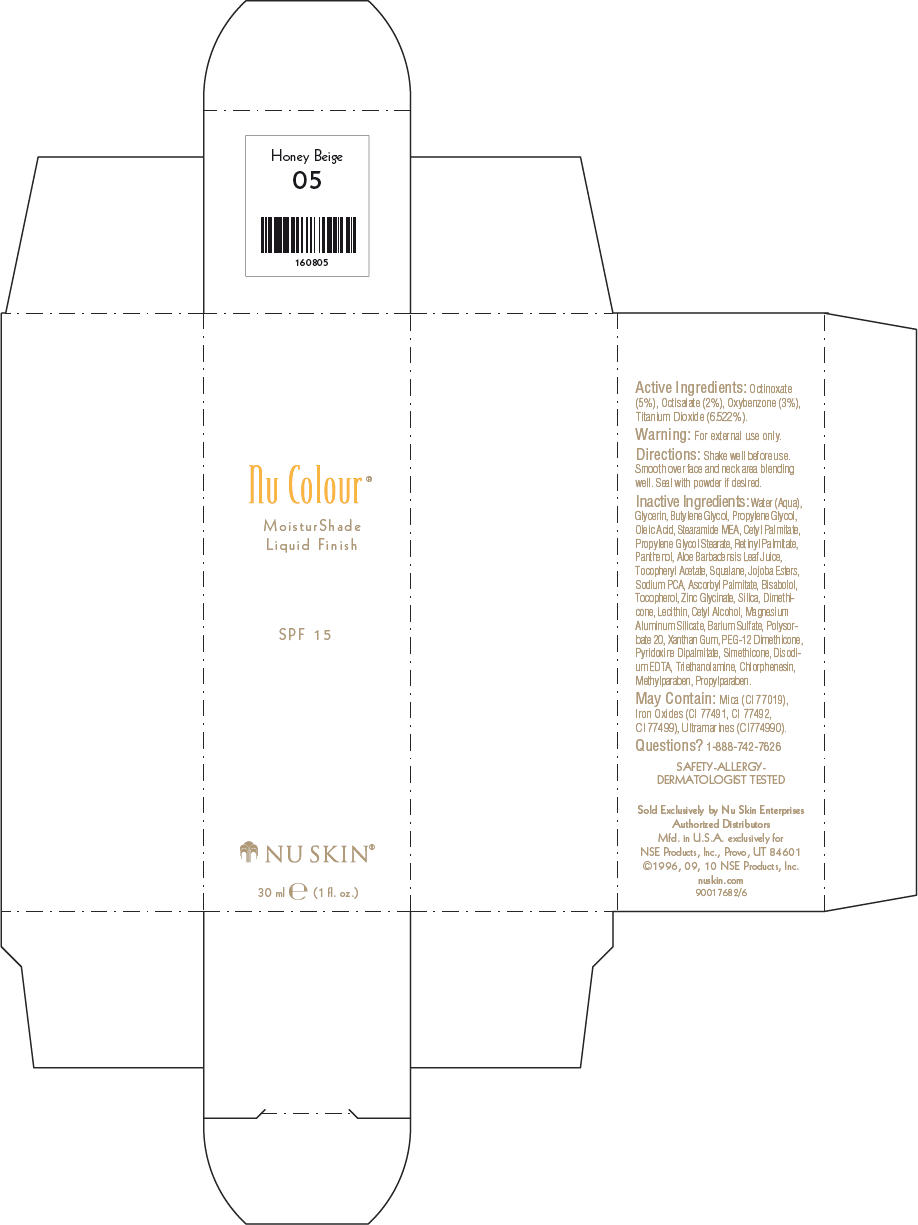
NU SKIN NU COLOUR MOISTURSHADE FINISH SPF 15 HONEY BEIGE- octinoxate, octisalate, oxybenzone, and titanium dioxide lotion
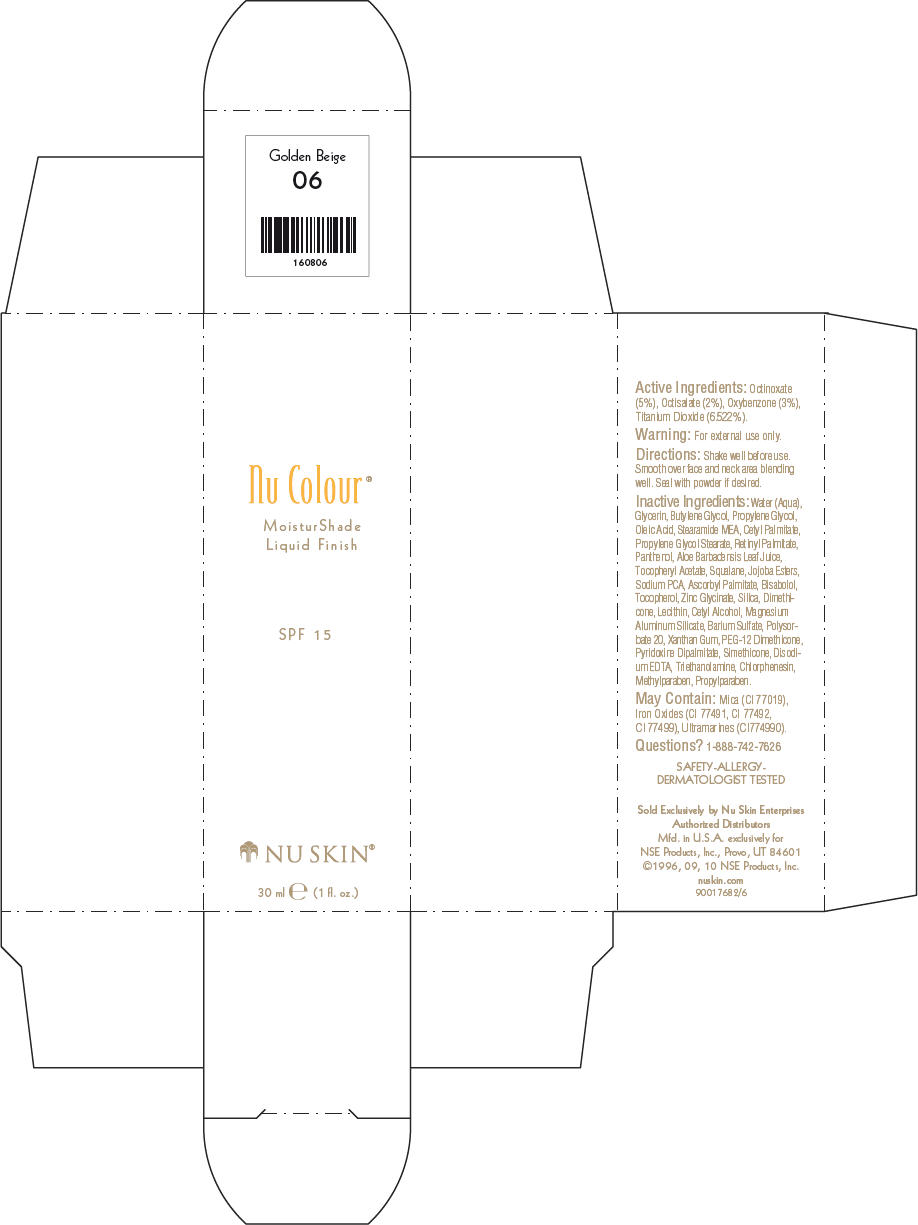
NU SKIN NU COLOUR MOISTURSHADE FINISH SPF 15 GOLDEN BEIGE- octinoxate, octisalate, oxybenzone, and titanium dioxide lotion
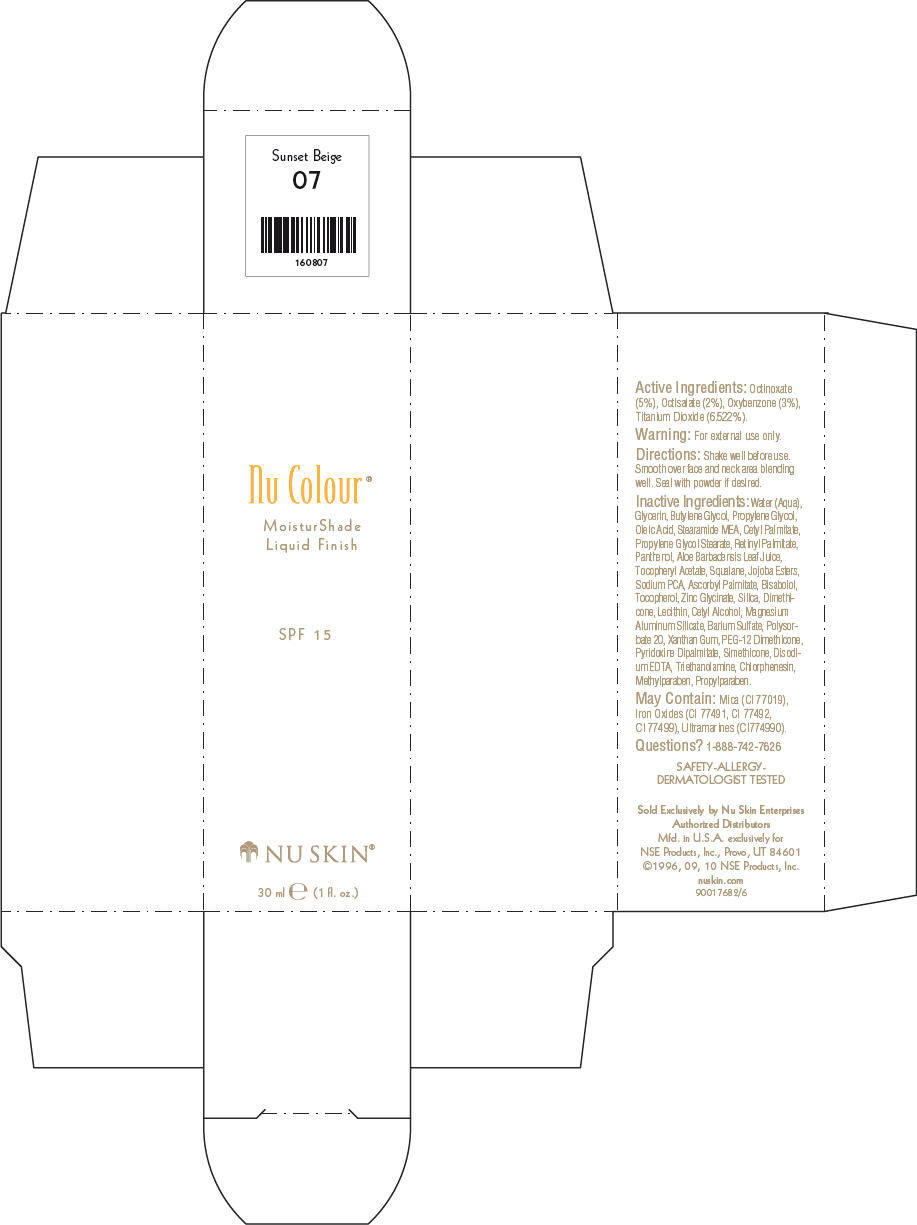
NU SKIN NU COLOUR MOISTURSHADE FINISH SPF 15 SUNSET BEIGE- octinoxate, octisalate, oxybenzone, and titanium dioxide lotion
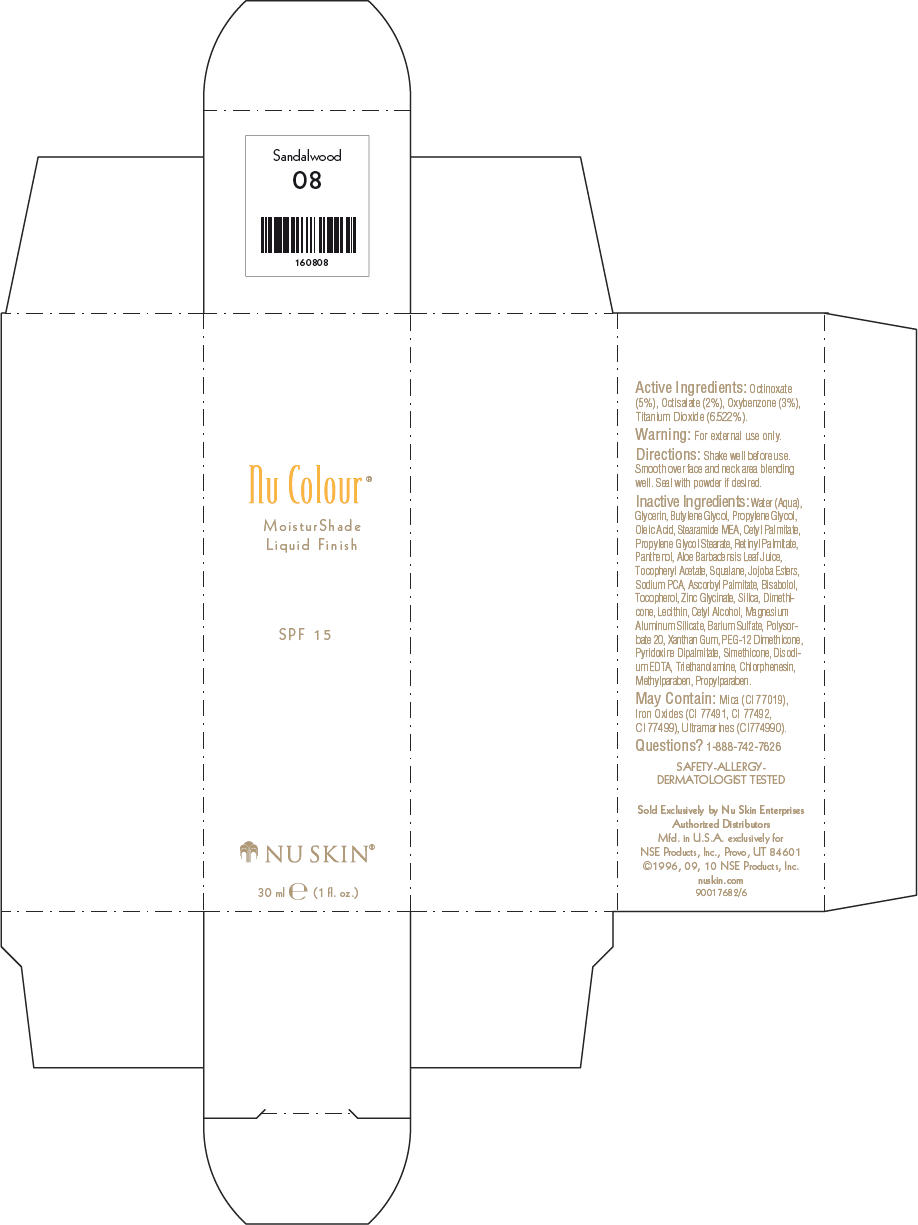
NU SKIN NU COLOUR MOISTURSHADE FINISH SPF 15 SANDALWOOD- octinoxate, octisalate, oxybenzone, and titanium dioxide lotion
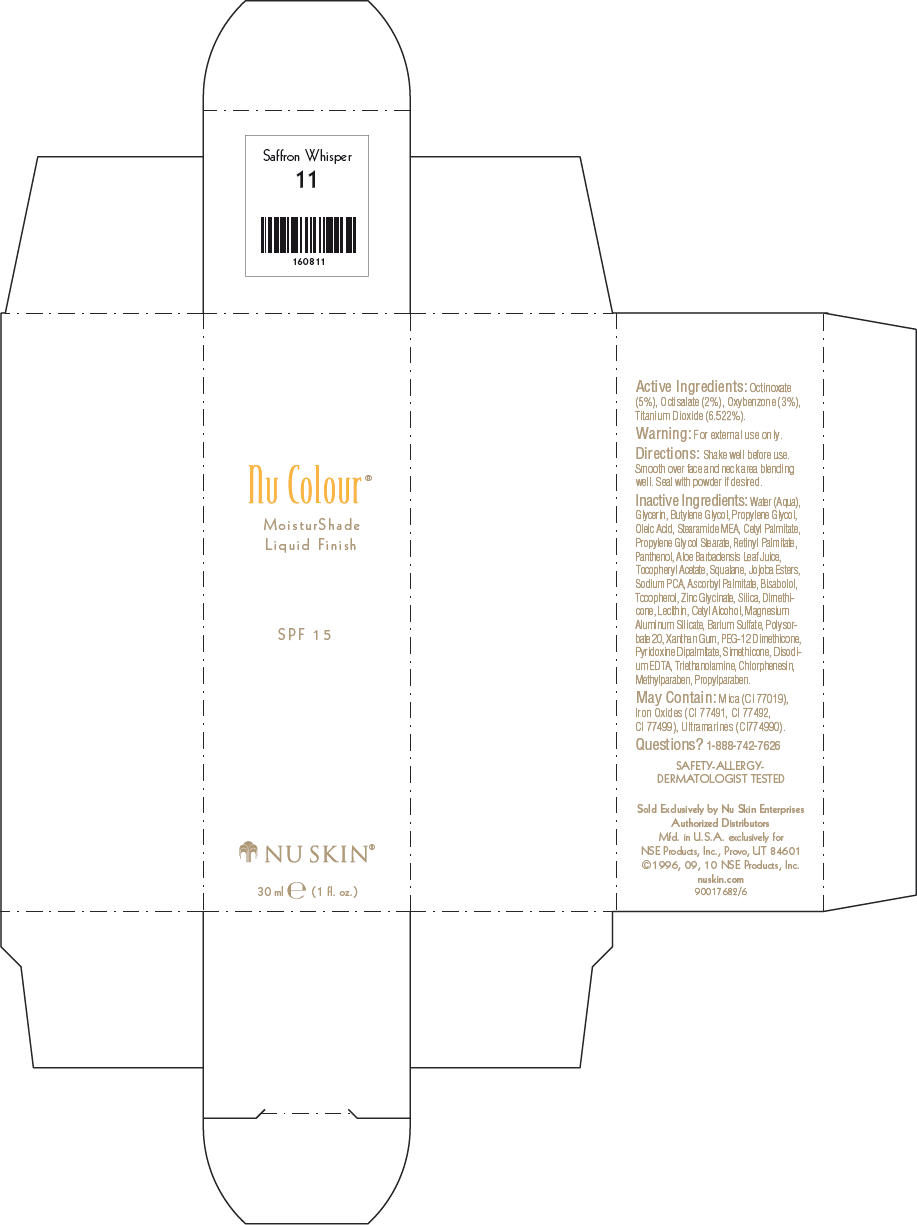
NU SKIN NU COLOUR MOISTURSHADE FINISH SPF 15 SAFFRON WHISPER- octinoxate, octisalate, oxybenzone, and titanium dioxide lotion
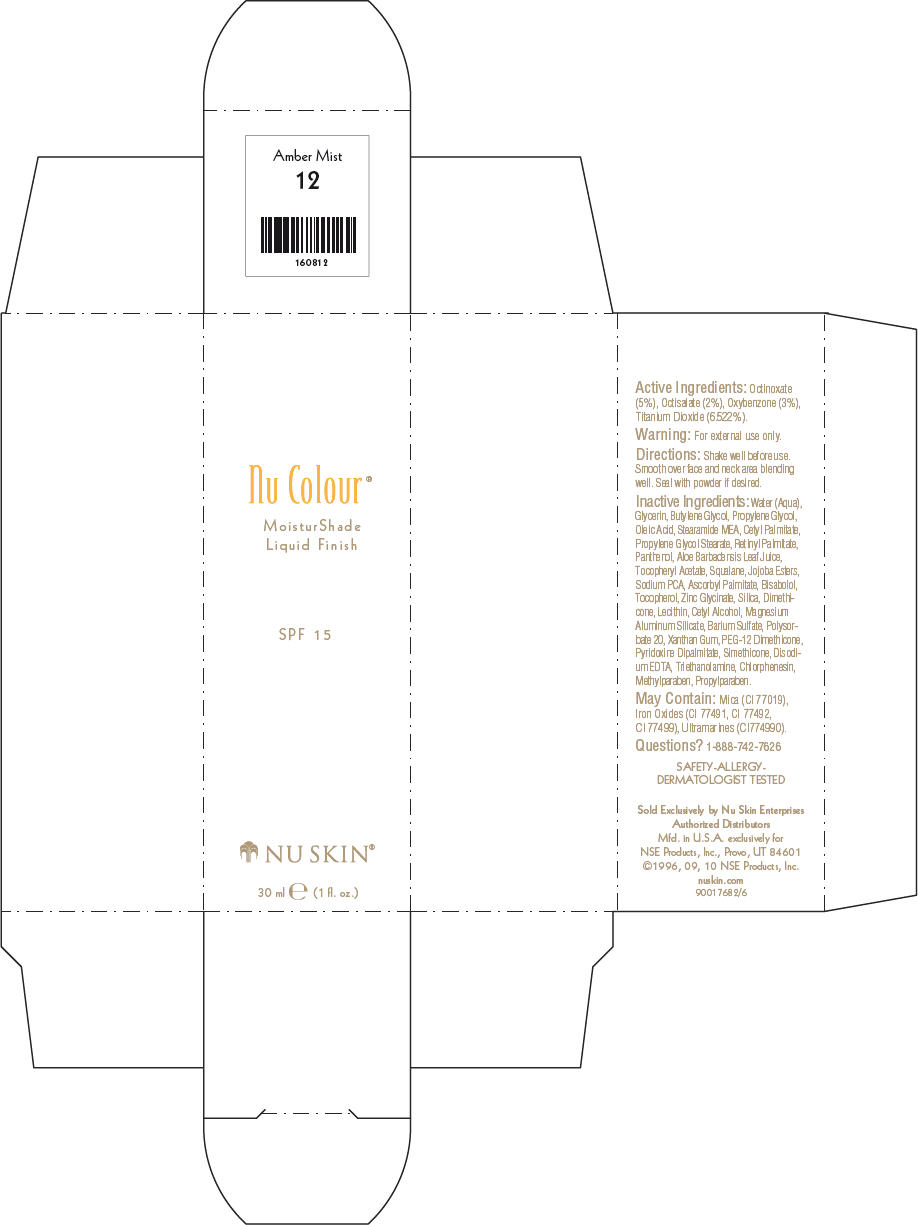
NU SKIN NU COLOUR MOISTURSHADE FINISH SPF 15 AMBER MIST- octinoxate, octisalate, oxybenzone, and titanium dioxide lotion
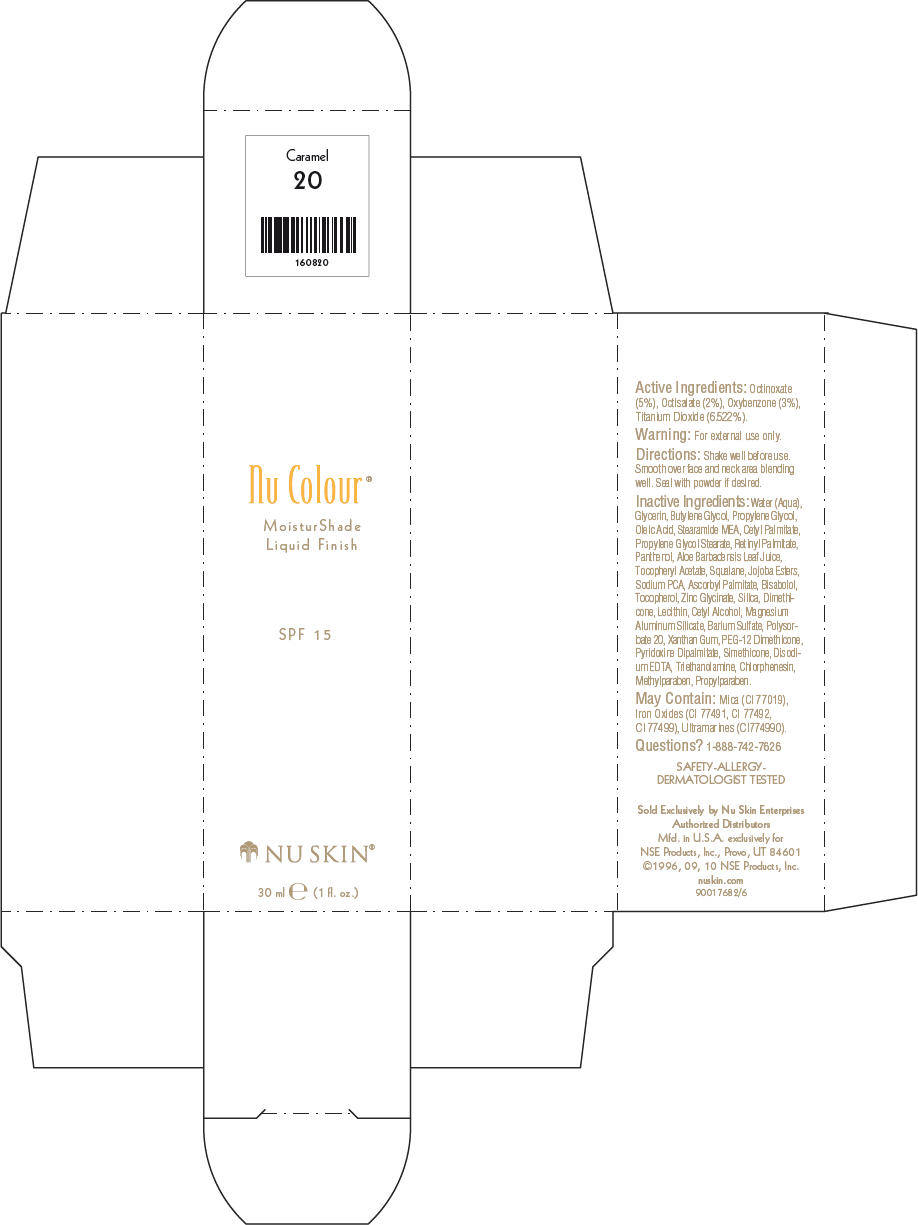
NU SKIN NU COLOUR MOISTURSHADE FINISH SPF 15 CARAMEL- octinoxate, octisalate, oxybenzone, and titanium dioxide lotion
NSE Products, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active Ingredients
Octinoxate (5%), Octisalate (2%), Oxybenzone (3%), Titanium Dioxide (6.522%).
Warning
For external use only.
Directions
Shake well before use. Smooth over face and neck area blending well. Seal with powder if desired.
Inactive Ingredients
Water (Aqua), Glycerin, Butylene Glycol, Propylene Glycol, Oleic Acid, Stearamide MEA, Cetyl Palmitate, Propylene Glycol Stearate, Retinyl Palmitate, Panthenol, Aloe Barbadensis Leaf Juice, Tocopheryl Acetate, Squalane, Jojoba Esters, Sodium PCA, Ascorbyl Palmitate, Bisabolol, Tocopherol, Zinc Glycinate, Silica, Dimethicone, Lecithin, Cetyl Alcohol, Magnesium Aluminum Silicate, Barium Sulfate, Polysorbate 20, Xanthan Gum, PEG-12 Dimethicone, Pyridoxine Dipalmitate, Simethicone, Disodium EDTA, Triethanolamine, Chlorphenesin, Methylparaben, Propylparaben.
May Contain
Mica (CI 77019), Iron Oxides (CI 77491, CI 77492, CI 77499), Ultramarines (CI774990).
Questions?
1-888-742-7626
PRINCIPAL DISPLAY PANEL - 30 ml Delicate Ivory Carton
Nu Colour®
MoisturShade
Liquid Finish
SPF 15
NU SKIN®
30 ml e (1 fl. oz.)
PRINCIPAL DISPLAY PANEL - 30 ml Shell Rose Carton
Nu Colour®
MoisturShade
Liquid Finish
SPF 15
NU SKIN®
30 ml e (1 fl. oz.)
PRINCIPAL DISPLAY PANEL - 30 ml Natural Beige Carton
Nu Colour®
MoisturShade
Liquid Finish
SPF 15
NU SKIN®
30 ml e (1 fl. oz.)
PRINCIPAL DISPLAY PANEL - 30 ml Honey Beige Carton
Nu Colour®
MoisturShade
Liquid Finish
SPF 15
NU SKIN®
30 ml e (1 fl. oz.)
PRINCIPAL DISPLAY PANEL - 30 ml Golden Beige Carton
Nu Colour®
MoisturShade
Liquid Finish
SPF 15
NU SKIN®
30 ml e (1 fl. oz.)
PRINCIPAL DISPLAY PANEL - 30 ml Sunset Beige Carton
Nu Colour®
MoisturShade
Liquid Finish
SPF 15
NU SKIN®
30 ml e (1 fl. oz.)
PRINCIPAL DISPLAY PANEL - 30 ml Sandalwood Carton
Nu Colour®
MoisturShade
Liquid Finish
SPF 15
NU SKIN®
30 ml e (1 fl. oz.)
PRINCIPAL DISPLAY PANEL - 30 ml Saffron Whisper Carton
Nu Colour®
MoisturShade
Liquid Finish
SPF 15
NU SKIN®
30 ml e (1 fl. oz.)
PRINCIPAL DISPLAY PANEL - 30 ml Amber Mist Carton
Nu Colour®
MoisturShade
Liquid Finish
SPF 15
NU SKIN®
30 ml e (1 fl. oz.)
PRINCIPAL DISPLAY PANEL - 30 ml Caramel Carton
Nu Colour®
MoisturShade
Liquid Finish
SPF 15
NU SKIN®
30 ml e (1 fl. oz.)
NSE Products, Inc.