Label: STUDIO FIX FLUID BROAD SPECTRUM SPF 15- octinoxate, titanium dioxide emulsion
- NDC Code(s): 40046-0082-1
- Packager: MAKEUP ART COSMETICS
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 17, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
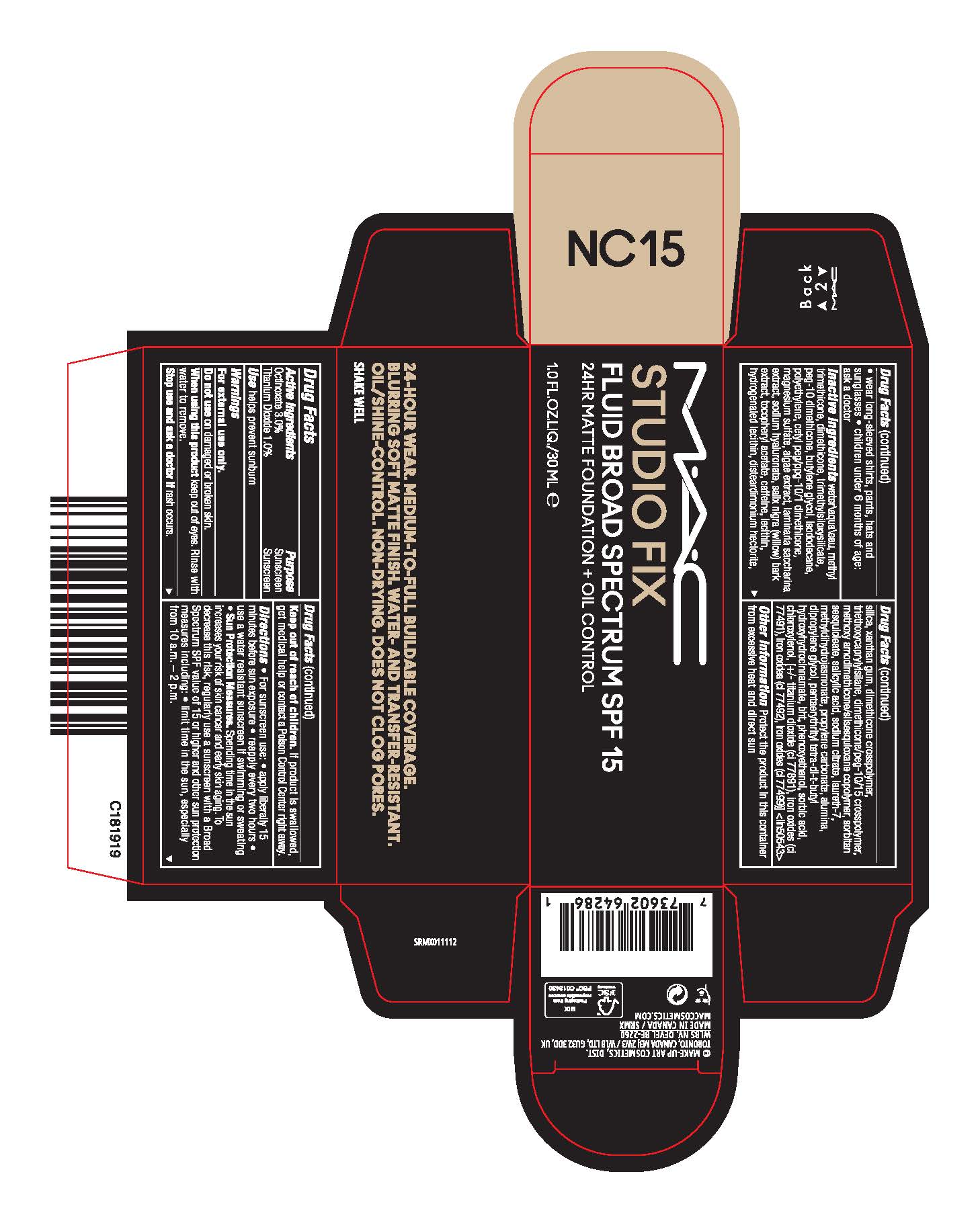
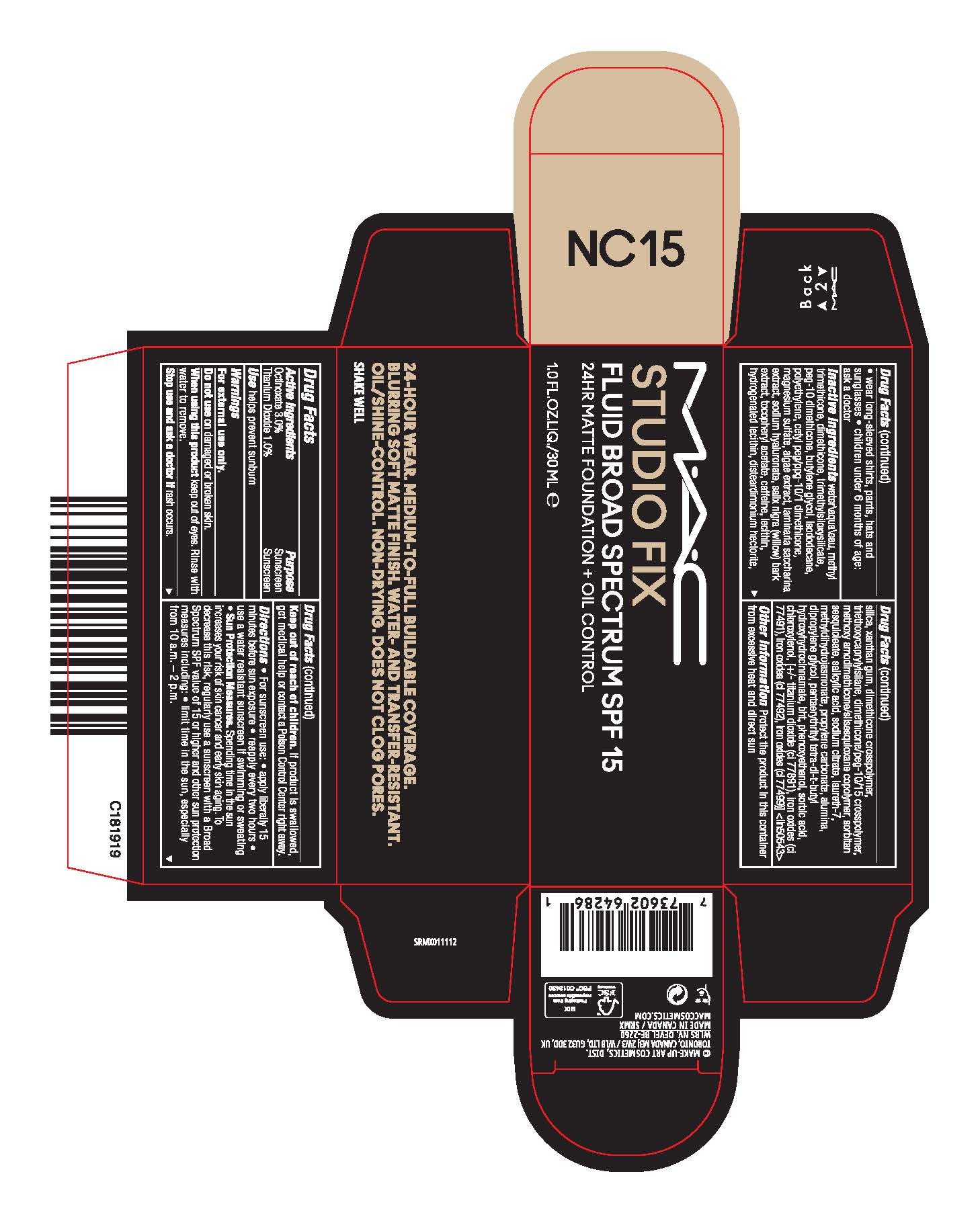
- Active Ingredients
- Purpose
- USE
- WARNINGS
-
Directions
- For sunscreen use:
- apply liberally 15 minutes before sun exposure
- reapply at least every two hours
- use a water resistant sunscreen if swimming or sweating
- Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m.–2 p.m.
- wear long-sleeved shirts, pants, hats and sunglasses
- children under 6 months of age: ask a doctor
-
Inactive Ingredients
WATER\AQUA\EAU,METHYL TRIMETHICONE,DIMETHICONE,TRIMETHYLSILOXYSILICATE,PEG-10 DIMETHICONE,BUTYLENE GLYCOL,ISODODECANE,POLYETHYLENE,CETYL PEG/PPG-10/1 DIMETHICONE,MAGNESIUM SULFATE,ALGAE EXTRACT,LAMINARIA SACCHARINA EXTRACT,SODIUM HYALURONATE,SALIX NIGRA (WILLOW) BARK EXTRACT,TOCOPHERYL ACETATE,CAFFEINE,LECITHIN,HYDROGENATED LECITHIN,DISTEARDIMONIUM HECTORITE,SILICA,XANTHAN GUM,DIMETHICONE CROSSPOLYMER,TRIETHOXYCAPRYLYLSILANE,DIMETHICONE/PEG-10/15 CROSSPOLYMER,METHOXY AMODIMETHICONE/SILSESQUIOXANE COPOLYMER,SORBITAN SESQUIOLEATE,SALICYLIC ACID,SODIUM CITRATE,LAURETH-7,METHYLDIHYDROJASMONATE,PROPYLENE CARBONATE,ALUMINA,DIPROPYLENE GLYCOL,PENTAERYTHRITYL TETRA-DI-T-BUTYL HYDROXYHYDROCINNAMATE,BHT,PHENOXYETHANOL,SORBIC ACID,CHLOROXYLENOL, [+/- TITANIUM DIOXIDE (CI 77891),IRON OXIDES (CI 77491),IRON OXIDES (CI 77492),IRON OXIDES (CI 77499)] <ILN50543>
- Other Information
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
STUDIO FIX FLUID BROAD SPECTRUM SPF 15
octinoxate, titanium dioxide emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:40046-0082 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 30 mg in 1 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) ALUMINUM OXIDE (UNII: LMI26O6933) WATER (UNII: 059QF0KO0R) TRIMETHYLSILOXYSILICATE (M/Q 0.6-0.8) (UNII: 5041RX63GN) ISODODECANE (UNII: A8289P68Y2) SACCHARINA LATISSIMA (UNII: 68CMP2MB55) CHLOROXYLENOL (UNII: 0F32U78V2Q) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) MAGNESIUM SULFATE, UNSPECIFIED (UNII: DE08037SAB) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 5) (UNII: 035JKJ76MT) PORPHYRIDIUM PURPUREUM (UNII: K2P8K2558N) SALIX NIGRA BARK (UNII: QU52J3A5B3) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) METHYL DIHYDROJASMONATE (SYNTHETIC) (UNII: 3GW44CIE3Y) SORBIC ACID (UNII: X045WJ989B) XANTHAN GUM (UNII: TTV12P4NEE) HYALURONATE SODIUM (UNII: YSE9PPT4TH) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) DIMETHICONE/PEG-10/15 CROSSPOLYMER (UNII: 21AS8B1BSS) SORBITAN SESQUIOLEATE (UNII: 0W8RRI5W5A) SALICYLIC ACID (UNII: O414PZ4LPZ) SODIUM CITRATE (UNII: 1Q73Q2JULR) DIPROPYLENE GLYCOL (UNII: E107L85C40) FERROSOFERRIC OXIDE (UNII: XM0M87F357) FERRIC OXIDE RED (UNII: 1K09F3G675) DIMETHICONE (UNII: 92RU3N3Y1O) PROPYLENE CARBONATE (UNII: 8D08K3S51E) PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) CAFFEINE (UNII: 3G6A5W338E) LAURETH-7 (UNII: Z95S6G8201) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) PHENOXYETHANOL (UNII: HIE492ZZ3T) METHYL TRIMETHICONE (UNII: S73ZQI0GXM) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:40046-0082-1 1 in 1 CARTON 05/12/2023 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 05/12/2023 Labeler - MAKEUP ART COSMETICS (010597206) Registrant - Estee Lauder Companies Inc. (790802086) Establishment Name Address ID/FEI Business Operations Estee Lauder Cosmetics Ltd 202952982 manufacture(40046-0082) Establishment Name Address ID/FEI Business Operations Estee Lauder Cosmetics Ltd. 204132062 manufacture(40046-0082) , pack(40046-0082) , label(40046-0082)

 MAC
MAC