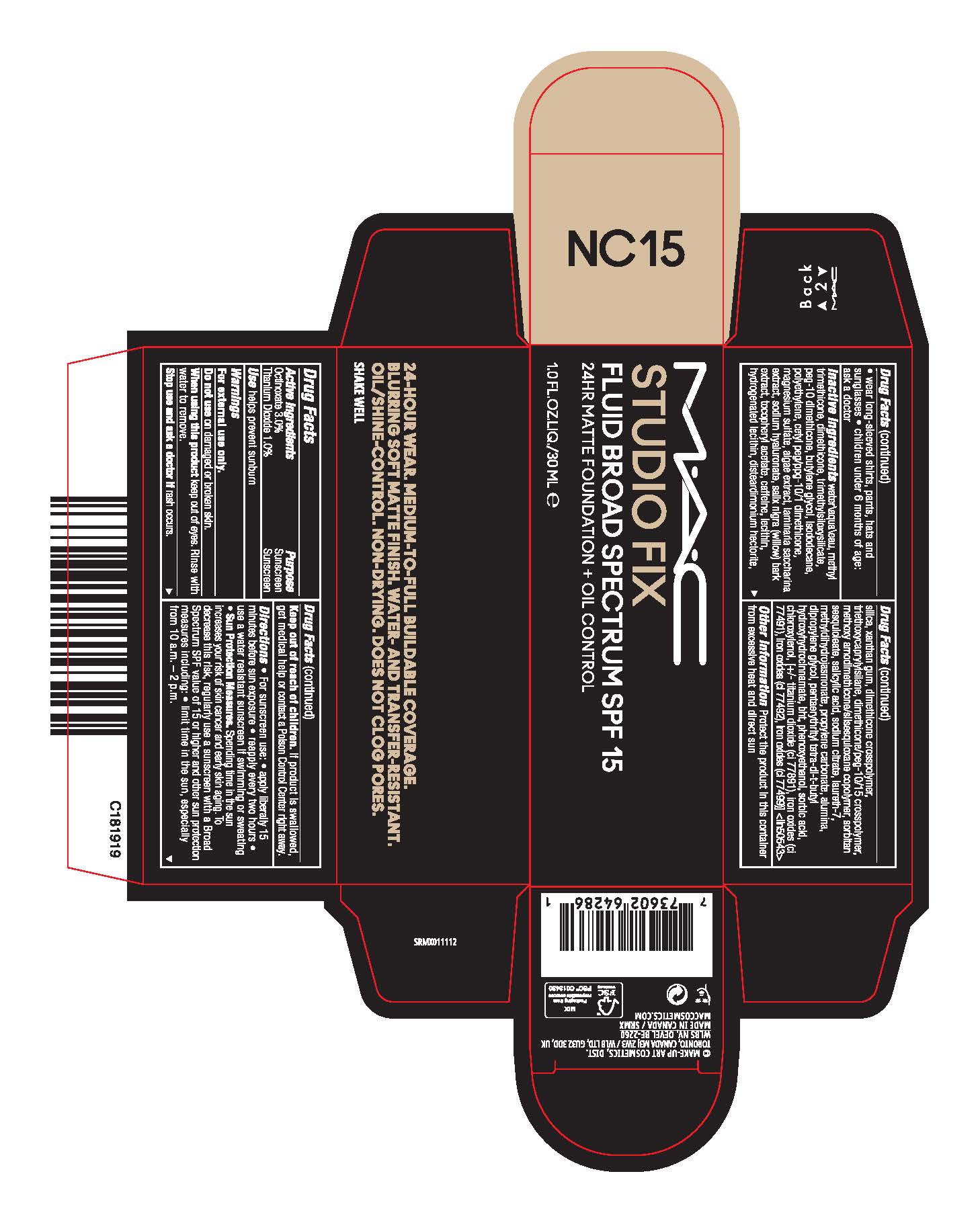
Directions
- For sunscreen use:
- apply liberally 15 minutes before sun exposure
- reapply at least every two hours
- use a water resistant sunscreen if swimming or sweating
- Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m.–2 p.m.
- wear long-sleeved shirts, pants, hats and sunglasses
- children under 6 months of age: ask a doctor
Inactive Ingredients
WATER\AQUA\EAU,METHYL TRIMETHICONE,DIMETHICONE,TRIMETHYLSILOXYSILICATE,PEG-10 DIMETHICONE,BUTYLENE GLYCOL,ISODODECANE,POLYETHYLENE,CETYL PEG/PPG-10/1 DIMETHICONE,MAGNESIUM SULFATE,ALGAE EXTRACT,LAMINARIA SACCHARINA EXTRACT,SODIUM HYALURONATE,SALIX NIGRA (WILLOW) BARK EXTRACT,TOCOPHERYL ACETATE,CAFFEINE,LECITHIN,HYDROGENATED LECITHIN,DISTEARDIMONIUM HECTORITE,SILICA,XANTHAN GUM,DIMETHICONE CROSSPOLYMER,TRIETHOXYCAPRYLYLSILANE,DIMETHICONE/PEG-10/15 CROSSPOLYMER,METHOXY AMODIMETHICONE/SILSESQUIOXANE COPOLYMER,SORBITAN SESQUIOLEATE,SALICYLIC ACID,SODIUM CITRATE,LAURETH-7,METHYLDIHYDROJASMONATE,PROPYLENE CARBONATE,ALUMINA,DIPROPYLENE GLYCOL,PENTAERYTHRITYL TETRA-DI-T-BUTYL HYDROXYHYDROCINNAMATE,BHT,PHENOXYETHANOL,SORBIC ACID,CHLOROXYLENOL, [+/- TITANIUM DIOXIDE (CI 77891),IRON OXIDES (CI 77491),IRON OXIDES (CI 77492),IRON OXIDES (CI 77499)] <ILN50543>
 MAC
MAC