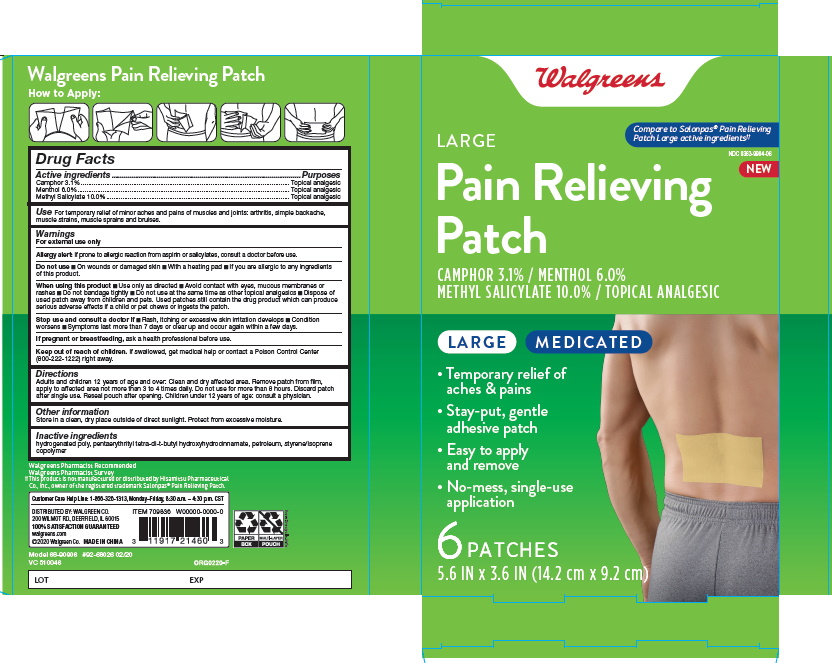
Label: LARGE PAIN RELIEVING- camphor menthol methyl salicytate patch
- NDC Code(s): 0363-9904-06
- Packager: Walgreen Company
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 18, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- INACTIVE INGREDIENThydrogenated poly, pentaerythrityl tetra-di-t-butyl hydroxyhydrocinnamate, petroleum, styrene/isoprene copolymer
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
- WARNINGS
- DOSAGE & ADMINISTRATION
- If pregnant or breastfeeding
- PURPOSE
-
When using this product:
■ Use only as directed ■ Avoid contact with eyes, mucous membranes or rashes ■ Do not bandage tightly ■ Do not use at the same time as other topical analgesics ■ Dispose of used patch away from children and pets. Used patches still contain the drug product which can produce serious adverse effects if a child or pet chews or ingests the patch.
- Do not use
- Stop use and consult a doctor
- Storage and Care
- Pain Relieving Path
-
INGREDIENTS AND APPEARANCE
LARGE PAIN RELIEVING
camphor menthol methyl salicytate patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0363-9904 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAMPHOR (SYNTHETIC) (UNII: 5TJD82A1ET) (CAMPHOR (SYNTHETIC) - UNII:5TJD82A1ET) CAMPHOR (SYNTHETIC) 3.1 g in 100 g MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 6 g in 100 g METHYL SALICYLATE (UNII: LAV5U5022Y) (SALICYLIC ACID - UNII:O414PZ4LPZ) METHYL SALICYLATE 10 g in 100 g Inactive Ingredients Ingredient Name Strength HYDROGENATED POLY(C6-14 OLEFIN; 2 CST) (UNII: P0TX083987) PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) LIQUID PETROLEUM (UNII: 6ZAE7X688J) STYRENE (UNII: 44LJ2U959V) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0363-9904-06 6 in 1 BOX 01/01/2020 1 9 g in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 01/01/2020 Labeler - Walgreen Company (008965063) Establishment Name Address ID/FEI Business Operations Foshan Aqua Gel Biotech Co.,Ltd. 529128763 manufacture(0363-9904)