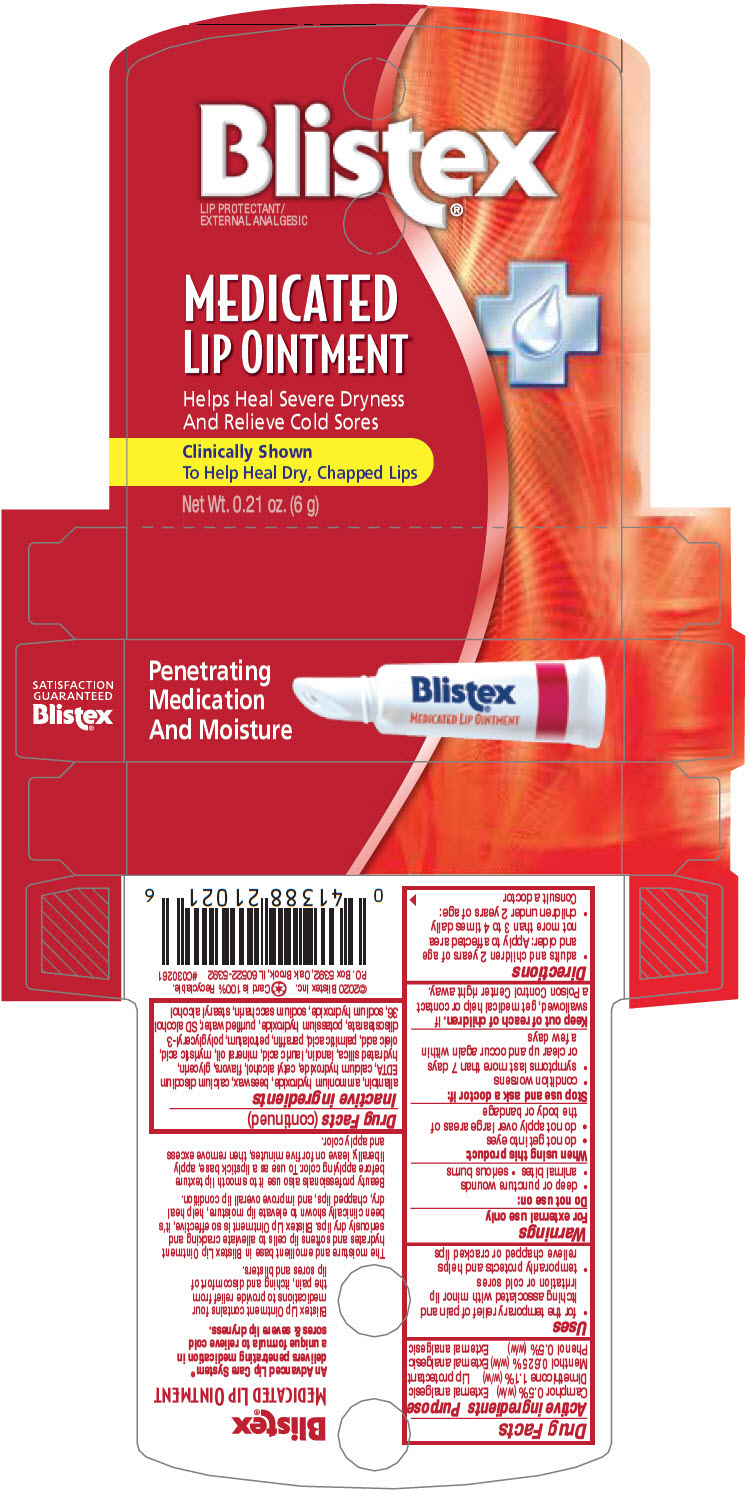
Label: BLISTEX MEDICATED (dimethicone, camphor- synthetic, menthol, and phenol ointment
-
NDC Code(s):
10157-9951-1,
10157-9951-2,
10157-9951-3,
10157-9951-4, view more10157-9951-5, 10157-9951-6, 10157-9951-7, 10157-9951-8
- Packager: Blistex Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 11, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Uses
- Warnings
- Directions
-
Inactive ingredients
allantoin, ammonium hydroxide, beeswax, calcium disodium EDTA, calcium hydroxide, cetyl alcohol, flavors, glycerin, hydrated silica, lanolin, lauric acid, mineral oil, myristic acid, oleic acid, palmitic acid, paraffin, petrolatum, polyglyceryl-3 diisostearate, potassium hydroxide, purified water, SD alcohol 36, sodium hydroxide, sodium saccharin, stearyl alcohol
- PRINCIPAL DISPLAY PANEL - 6 g Tube Carton
-
INGREDIENTS AND APPEARANCE
BLISTEX MEDICATED
dimethicone, camphor (synthetic), menthol, and phenol ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10157-9951 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Dimethicone (UNII: 92RU3N3Y1O) (Dimethicone - UNII:92RU3N3Y1O) Dimethicone 1.1 g in 100 g Camphor (synthetic) (UNII: 5TJD82A1ET) (Camphor (synthetic) - UNII:5TJD82A1ET) Camphor (synthetic) 0.5 g in 100 g Menthol, unspecified form (UNII: L7T10EIP3A) (Menthol, unspecified form - UNII:L7T10EIP3A) Menthol, unspecified form 0.625 g in 100 g Phenol (UNII: 339NCG44TV) (Phenol - UNII:339NCG44TV) Phenol 0.5 g in 100 g Inactive Ingredients Ingredient Name Strength allantoin (UNII: 344S277G0Z) ammonia (UNII: 5138Q19F1X) yellow wax (UNII: 2ZA36H0S2V) edetate calcium disodium anhydrous (UNII: 8U5D034955) calcium hydroxide (UNII: PF5DZW74VN) cetyl alcohol (UNII: 936JST6JCN) glycerin (UNII: PDC6A3C0OX) hydrated silica (UNII: Y6O7T4G8P9) lanolin (UNII: 7EV65EAW6H) lauric acid (UNII: 1160N9NU9U) mineral oil (UNII: T5L8T28FGP) myristic acid (UNII: 0I3V7S25AW) oleic acid (UNII: 2UMI9U37CP) palmitic acid (UNII: 2V16EO95H1) paraffin (UNII: I9O0E3H2ZE) petrolatum (UNII: 4T6H12BN9U) polyglyceryl-3 diisostearate (UNII: 46P231IQV8) potassium hydroxide (UNII: WZH3C48M4T) water (UNII: 059QF0KO0R) sodium hydroxide (UNII: 55X04QC32I) saccharin sodium (UNII: SB8ZUX40TY) stearyl alcohol (UNII: 2KR89I4H1Y) Product Characteristics Color WHITE Score Shape Size Flavor MINT Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10157-9951-1 6 g in 1 TUBE; Type 0: Not a Combination Product 12/01/2000 2 NDC:10157-9951-3 10 g in 1 TUBE; Type 0: Not a Combination Product 12/01/2000 3 NDC:10157-9951-4 2.25 g in 1 TUBE; Type 0: Not a Combination Product 12/01/2000 4 NDC:10157-9951-5 8 g in 1 TUBE; Type 0: Not a Combination Product 12/01/2000 5 NDC:10157-9951-2 1 in 1 CARTON 12/01/2000 5 NDC:10157-9951-6 6 g in 1 TUBE; Type 0: Not a Combination Product 6 NDC:10157-9951-7 3 in 1 CARTON 12/01/2000 6 6 g in 1 TUBE; Type 0: Not a Combination Product 7 NDC:10157-9951-8 4 in 1 CARTON 12/01/2000 7 6 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M017 12/01/2000 Labeler - Blistex Inc. (005126354) Establishment Name Address ID/FEI Business Operations Blistex Inc. 005126354 MANUFACTURE(10157-9951)