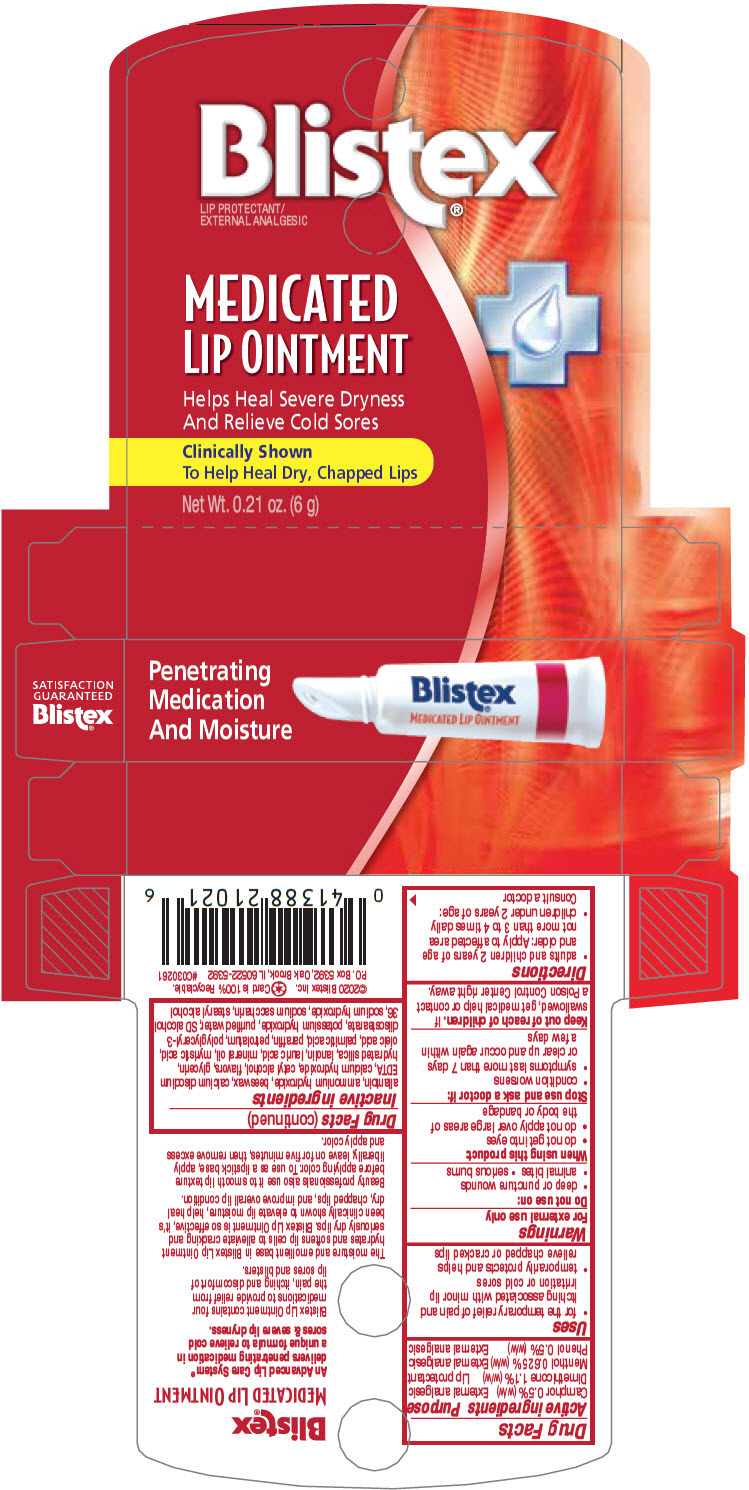
BLISTEX MEDICATED- dimethicone, camphor (synthetic), menthol, and phenol ointment
Blistex Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
| Active ingredients | Purpose |
| Camphor 0.5% (w/w) | External analgesic |
| Dimethicone 1.1% (w/w) | Lip protectant |
| Menthol 0.625% (w/w) | External analgesic |
| Phenol 0.5% (w/w) | External analgesic |
Uses
- for the temporary relief of pain and itching associated with minor lip irritation or cold sores
- temporarily protects and helps relieve chapped or cracked lips
Warnings
For external use only
Do not use on
- deep or puncture wounds
- animal bites
- serious burns
When using this product
- do not get into eyes
- do not apply over large areas of the body or bandage
Stop use and ask a doctor if
- condition worsens
- symptoms last more than 7 days or clear up and occur again within a few days
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- adults and children 2 years of age and older: Apply to affected area not more than 3 to 4 times daily
- children under 2 years of age: Consult a doctor
Inactive ingredients
allantoin, ammonium hydroxide, beeswax, calcium disodium EDTA, calcium hydroxide, cetyl alcohol, flavors, glycerin, hydrated silica, lanolin, lauric acid, mineral oil, myristic acid, oleic acid, palmitic acid, paraffin, petrolatum, polyglyceryl-3 diisostearate, potassium hydroxide, purified water, SD alcohol 36, sodium hydroxide, sodium saccharin, stearyl alcohol
PRINCIPAL DISPLAY PANEL - 6 g Tube Carton
Penetrating
Medication
And Moisture
Blistex®
MEDICATED LIP OINTMENT
Blistex Inc.