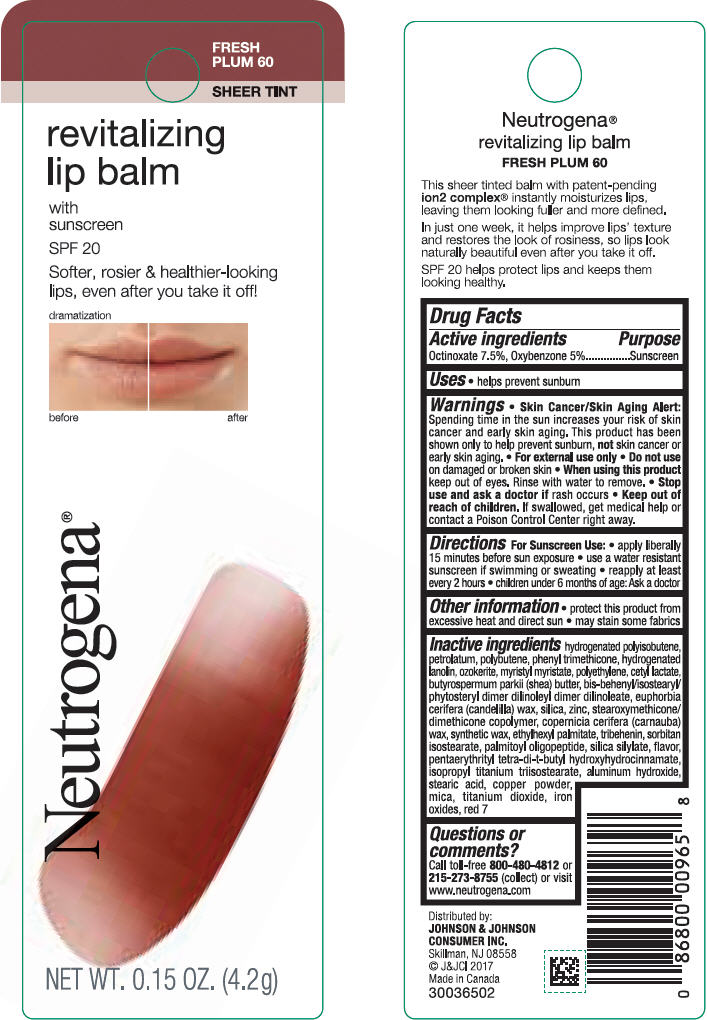
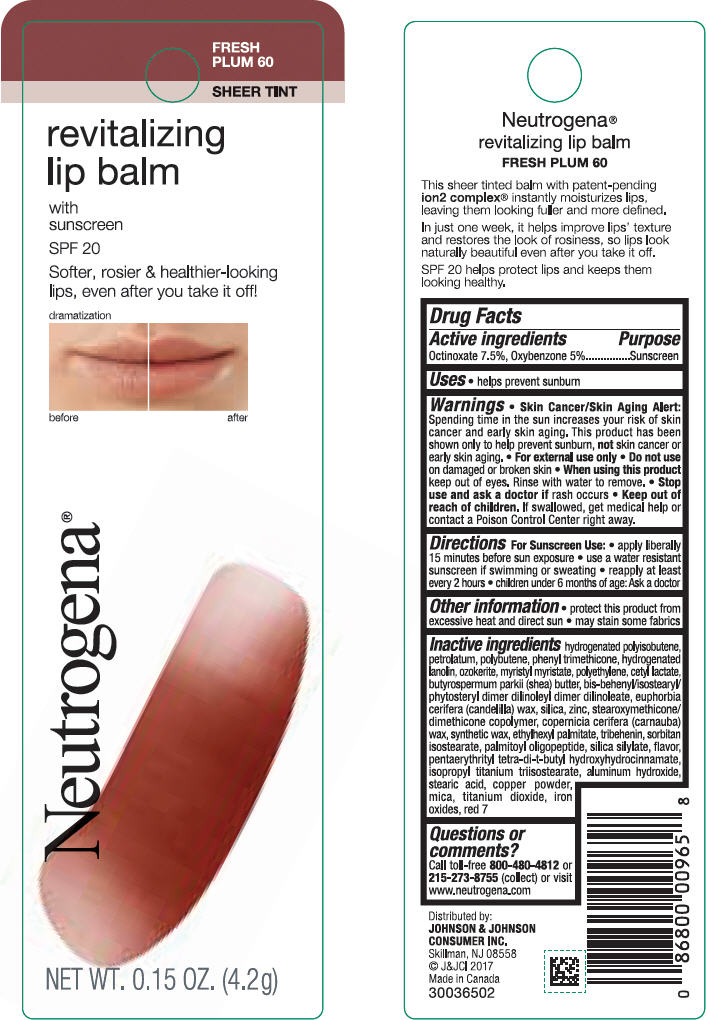
Label: NEUTROGENA REVITALIZING LIP BALM SPF 20 - FRESH PLUM 60- octinoxate and oxybenzone lipstick
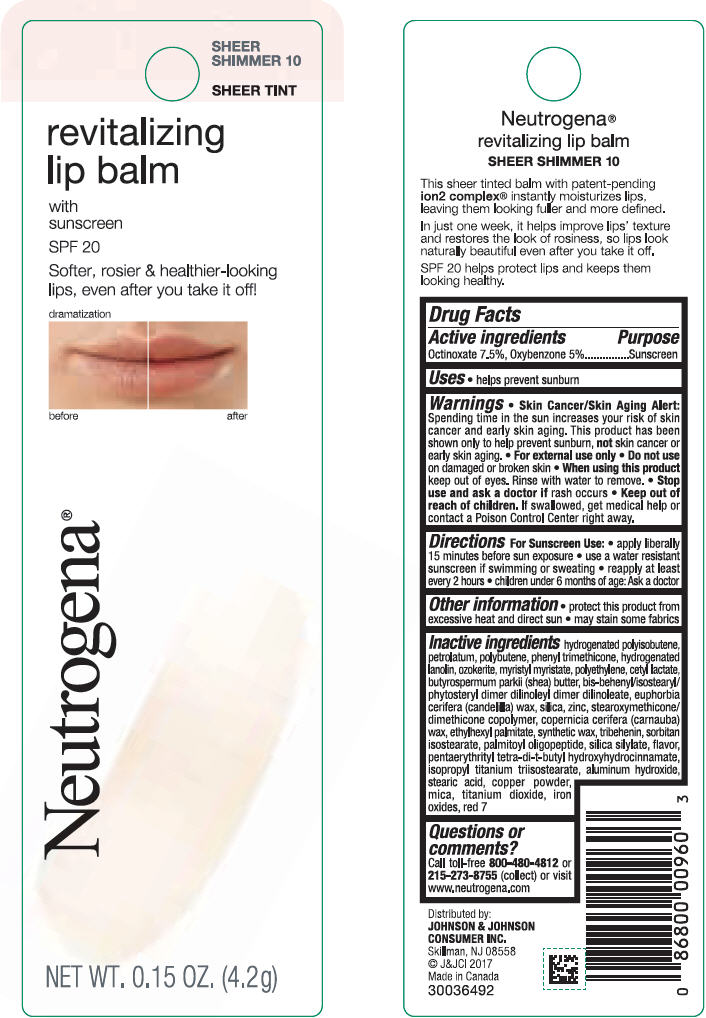
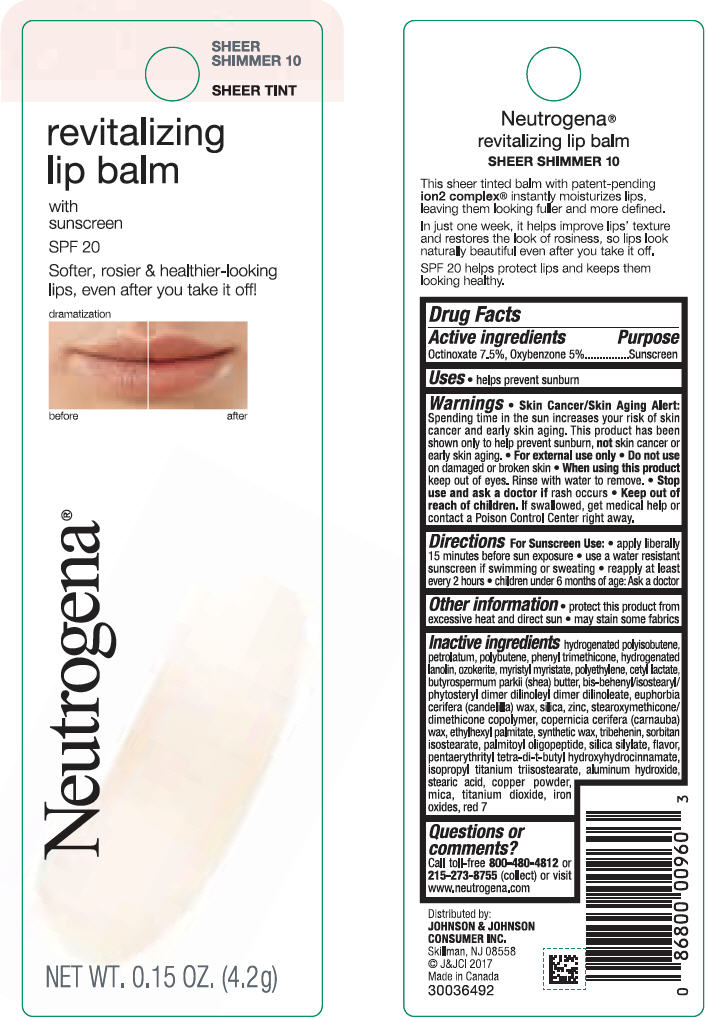
NEUTROGENA REVITALIZING LIP BALM SPF 20 - SHEEER SHIMMER 10- octinoxate and oxybenzone lipstick
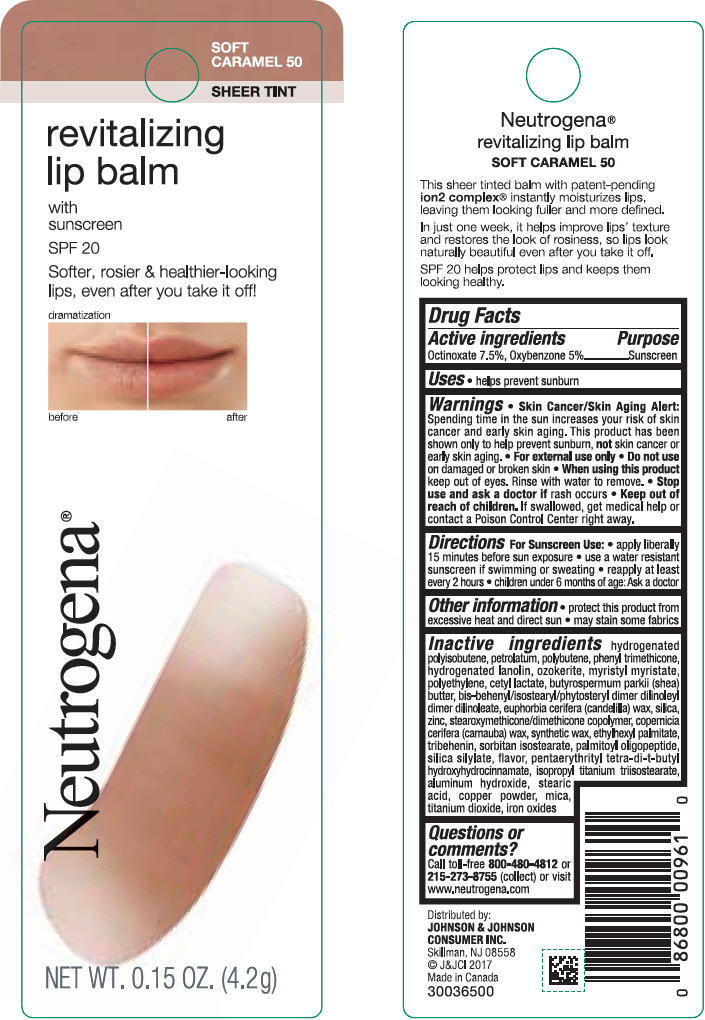
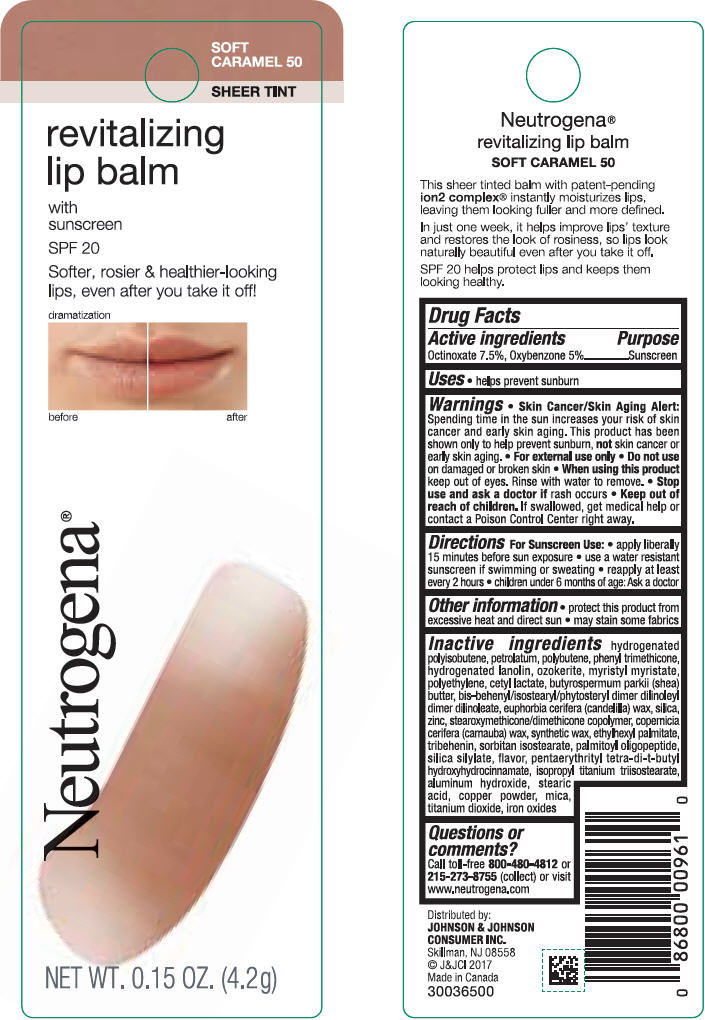
NEUTROGENA REVITALIZING LIP BALM SPF 20 - SOFT CARAMEL 50- octinoxate and oxybenzone lipstick
NEUTROGENA REVITALIZING LIP BALM SPF 20 - HEALTHY BLUSH 20- octinoxate and oxybenzone lipstick
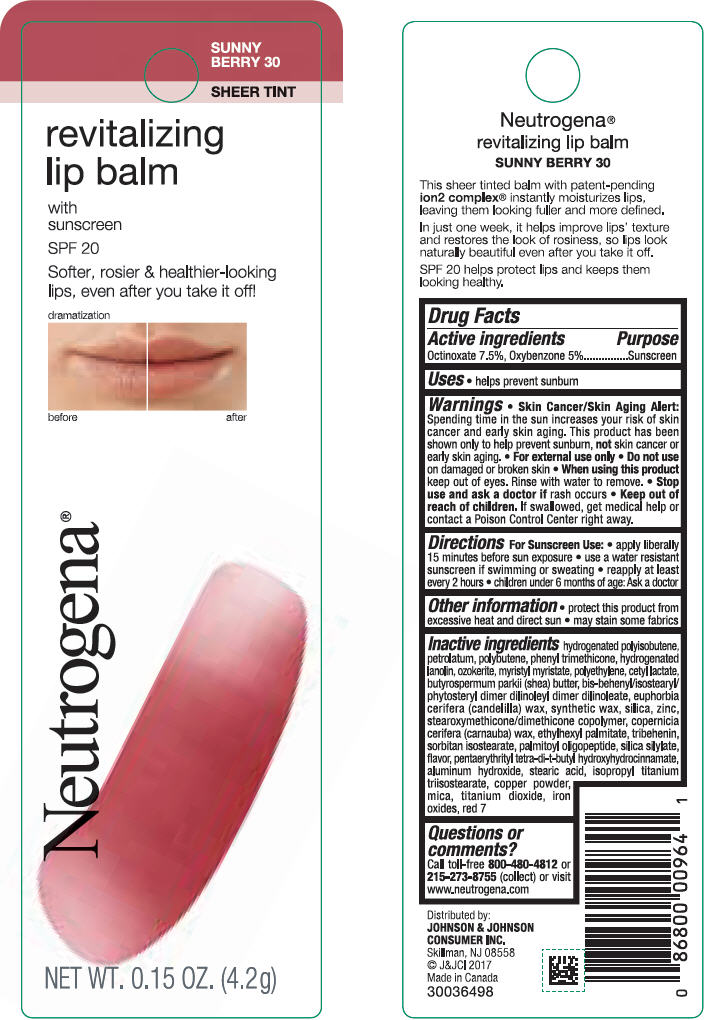
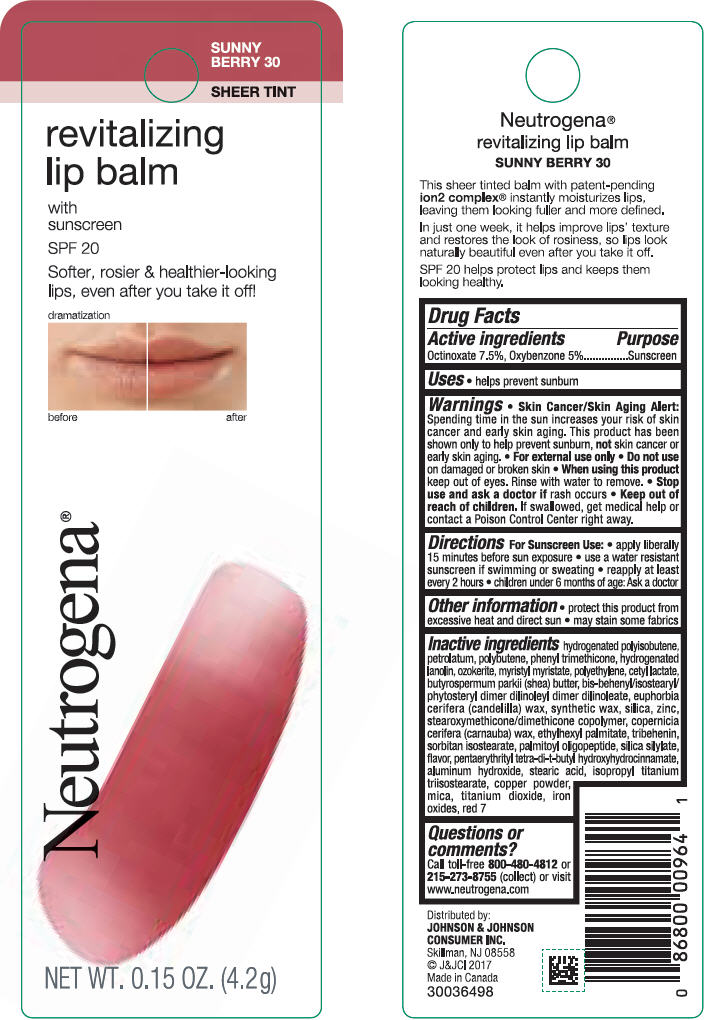
NEUTROGENA REVITALIZING LIP BALM SPF 20 - SUNNY BERRY 30- octinoxate and oxybenzone lipstick
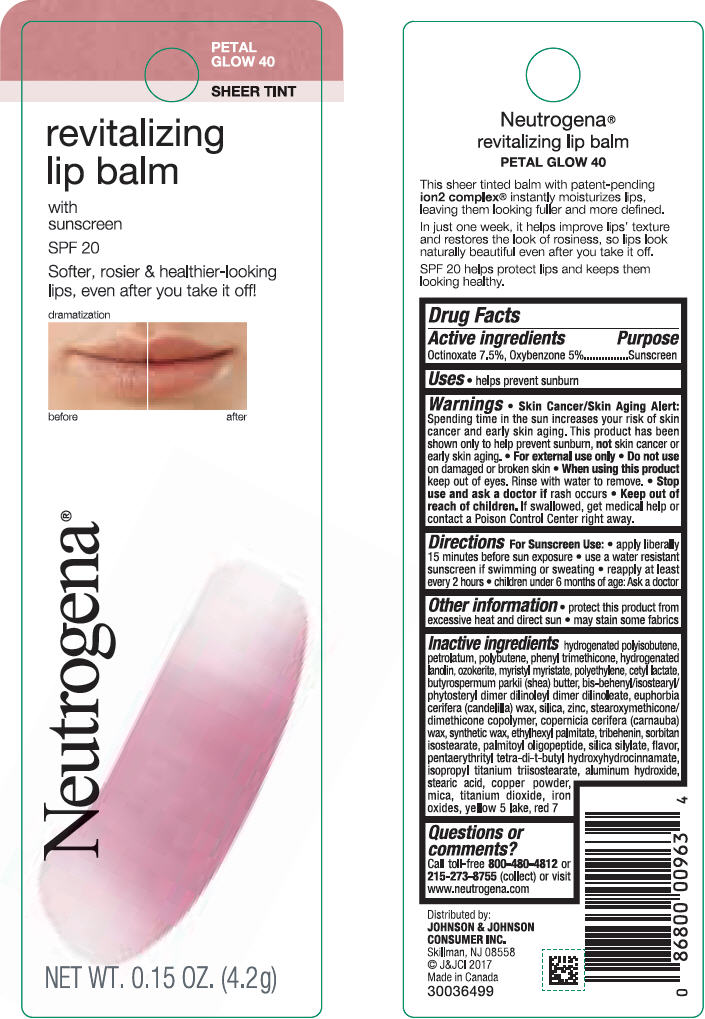
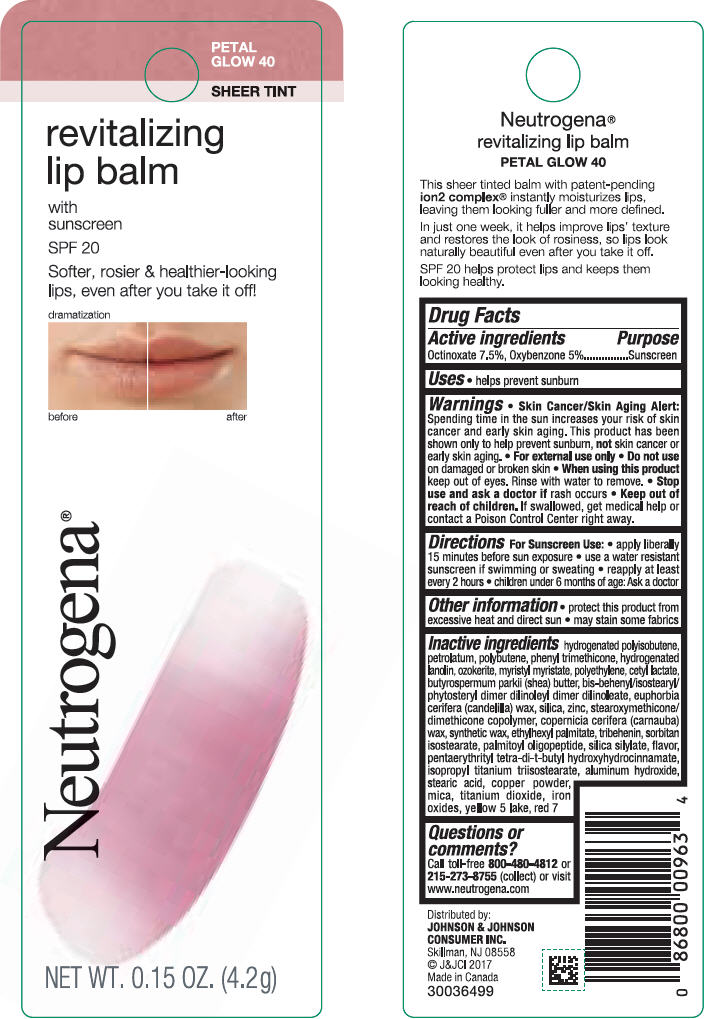
NEUTROGENA REVITALIZING LIP BALM SPF 20 - PETAL GLOW 40- octinoxate and oxybenzone lipstick
-
NDC Code(s):
69968-0251-1,
69968-0252-1,
69968-0253-1,
69968-0254-1, view more69968-0255-1, 69968-0256-1
- Packager: Kenvue Brands LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 11, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredients
- Purpose
- Uses
- Warnings
- Directions
- Other information
-
Inactive ingredients
hydrogenated polyisobutene, petrolatum, polybutene, phenyl trimethicone, hydrogenated lanolin, ozokerite, myristyl myristate, polyethylene, cetyl lactate, butyrospermum parkii (shea) butter, bis-behenyl/isostearyl/phytosteryl dimer dilinoleyl dimer dilinoleate, euphorbia cerifera (candelilla) wax, silica, zinc, stearoxymethicone/dimethicone copolymer, copernicia cerifera (carnauba) wax, synthetic wax, ethylhexyl palmitate, tribehenin, sorbitan isostearate, palmitoyl oligopeptide, silica silylate, flavor, pentaerythrityl tetra-di-t-butyl hydroxyhydrocinnamate, isopropyl titanium triisostearate, aluminum hydroxide, stearic acid, copper powder, mica, titanium dioxide, iron oxides, red 7
- Questions or comments?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 4.2 g Tube Blister Pack - Fresh Plum 60
- PRINCIPAL DISPLAY PANEL - 4.2 g Tube Blister Pack - Sheer Shimmer 10
- PRINCIPAL DISPLAY PANEL - 4.2 g Tube Blister Pack - Soft Caramel 50
- PRINCIPAL DISPLAY PANEL - 4.2 g Tube Blister Pack - Healthy Blush 20
- PRINCIPAL DISPLAY PANEL - 4.2 g Tube Blister Pack - Sunny Berry 30
- PRINCIPAL DISPLAY PANEL - 4.2 g Tube Blister Pack - Petal Glow 40
-
INGREDIENTS AND APPEARANCE
NEUTROGENA REVITALIZING LIP BALM SPF 20 - FRESH PLUM 60
octinoxate and oxybenzone lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0256 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 75 mg in 1 g OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 50 mg in 1 g Inactive Ingredients Ingredient Name Strength HYDROGENATED POLYBUTENE (1300 MW) (UNII: 7D1YQ9Y5EZ) PETROLATUM (UNII: 4T6H12BN9U) POLYBUTENE (1400 MW) (UNII: 1NA5AO9GH7) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) LANOLIN ALCOHOLS (UNII: 884C3FA9HE) CERESIN (UNII: Q1LS2UJO3A) MYRISTYL MYRISTATE (UNII: 4042ZC00DY) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CETYL LACTATE (UNII: A7EVH2RK4O) SHEA BUTTER (UNII: K49155WL9Y) CANDELILLA WAX (UNII: WL0328HX19) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) ZINC (UNII: J41CSQ7QDS) CARNAUBA WAX (UNII: R12CBM0EIZ) ETHYLHEXYL PALMITATE (UNII: 2865993309) TRIBEHENIN (UNII: 8OC9U7TQZ0) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) PALMITOYL TRIPEPTIDE-1 (UNII: RV743D216M) PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) ISOPROPYL TITANIUM TRIISOSTEARATE (UNII: 949E3KBJ1I) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) STEARIC ACID (UNII: 4ELV7Z65AP) COPPER (UNII: 789U1901C5) MICA (UNII: V8A1AW0880) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) D&C RED NO. 7 (UNII: ECW0LZ41X8) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0256-1 1 in 1 BLISTER PACK 11/01/2010 1 4.2 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 11/01/2010 NEUTROGENA REVITALIZING LIP BALM SPF 20 - SHEEER SHIMMER 10
octinoxate and oxybenzone lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0251 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 75 mg in 1 g OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 50 mg in 1 g Inactive Ingredients Ingredient Name Strength HYDROGENATED POLYBUTENE (1300 MW) (UNII: 7D1YQ9Y5EZ) PETROLATUM (UNII: 4T6H12BN9U) POLYBUTENE (1400 MW) (UNII: 1NA5AO9GH7) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) LANOLIN ALCOHOLS (UNII: 884C3FA9HE) CERESIN (UNII: Q1LS2UJO3A) MYRISTYL MYRISTATE (UNII: 4042ZC00DY) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CETYL LACTATE (UNII: A7EVH2RK4O) SHEA BUTTER (UNII: K49155WL9Y) CANDELILLA WAX (UNII: WL0328HX19) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) ZINC (UNII: J41CSQ7QDS) CARNAUBA WAX (UNII: R12CBM0EIZ) ETHYLHEXYL PALMITATE (UNII: 2865993309) TRIBEHENIN (UNII: 8OC9U7TQZ0) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) PALMITOYL TRIPEPTIDE-1 (UNII: RV743D216M) PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) ISOPROPYL TITANIUM TRIISOSTEARATE (UNII: 949E3KBJ1I) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) STEARIC ACID (UNII: 4ELV7Z65AP) COPPER (UNII: 789U1901C5) MICA (UNII: V8A1AW0880) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) D&C RED NO. 7 (UNII: ECW0LZ41X8) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0251-1 1 in 1 BLISTER PACK 11/01/2010 1 4.2 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 11/01/2010 NEUTROGENA REVITALIZING LIP BALM SPF 20 - SOFT CARAMEL 50
octinoxate and oxybenzone lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0252 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 75 mg in 1 g OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 50 mg in 1 g Inactive Ingredients Ingredient Name Strength HYDROGENATED POLYBUTENE (1300 MW) (UNII: 7D1YQ9Y5EZ) PETROLATUM (UNII: 4T6H12BN9U) POLYBUTENE (1400 MW) (UNII: 1NA5AO9GH7) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) LANOLIN ALCOHOLS (UNII: 884C3FA9HE) CERESIN (UNII: Q1LS2UJO3A) MYRISTYL MYRISTATE (UNII: 4042ZC00DY) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CETYL LACTATE (UNII: A7EVH2RK4O) SHEA BUTTER (UNII: K49155WL9Y) CANDELILLA WAX (UNII: WL0328HX19) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) ZINC (UNII: J41CSQ7QDS) CARNAUBA WAX (UNII: R12CBM0EIZ) ETHYLHEXYL PALMITATE (UNII: 2865993309) TRIBEHENIN (UNII: 8OC9U7TQZ0) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) PALMITOYL TRIPEPTIDE-1 (UNII: RV743D216M) PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) ISOPROPYL TITANIUM TRIISOSTEARATE (UNII: 949E3KBJ1I) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) STEARIC ACID (UNII: 4ELV7Z65AP) COPPER (UNII: 789U1901C5) MICA (UNII: V8A1AW0880) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0252-1 1 in 1 BLISTER PACK 11/01/2010 1 4.2 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 11/01/2010 NEUTROGENA REVITALIZING LIP BALM SPF 20 - HEALTHY BLUSH 20
octinoxate and oxybenzone lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0253 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 75 mg in 1 g OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 50 mg in 1 g Inactive Ingredients Ingredient Name Strength HYDROGENATED POLYBUTENE (1300 MW) (UNII: 7D1YQ9Y5EZ) PETROLATUM (UNII: 4T6H12BN9U) POLYBUTENE (1400 MW) (UNII: 1NA5AO9GH7) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) LANOLIN ALCOHOLS (UNII: 884C3FA9HE) CERESIN (UNII: Q1LS2UJO3A) MYRISTYL MYRISTATE (UNII: 4042ZC00DY) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CETYL LACTATE (UNII: A7EVH2RK4O) SHEA BUTTER (UNII: K49155WL9Y) CANDELILLA WAX (UNII: WL0328HX19) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) ZINC (UNII: J41CSQ7QDS) CARNAUBA WAX (UNII: R12CBM0EIZ) ETHYLHEXYL PALMITATE (UNII: 2865993309) TRIBEHENIN (UNII: 8OC9U7TQZ0) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) PALMITOYL TRIPEPTIDE-1 (UNII: RV743D216M) PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) ISOPROPYL TITANIUM TRIISOSTEARATE (UNII: 949E3KBJ1I) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) STEARIC ACID (UNII: 4ELV7Z65AP) COPPER (UNII: 789U1901C5) MICA (UNII: V8A1AW0880) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 6 (UNII: 481744AI4O) D&C RED NO. 7 (UNII: ECW0LZ41X8) STANNIC OXIDE (UNII: KM7N50LOS6) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0253-1 1 in 1 BLISTER PACK 11/01/2010 1 4.2 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 11/01/2010 NEUTROGENA REVITALIZING LIP BALM SPF 20 - SUNNY BERRY 30
octinoxate and oxybenzone lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0255 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 75 mg in 1 g OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 50 mg in 1 g Inactive Ingredients Ingredient Name Strength D&C RED NO. 7 (UNII: ECW0LZ41X8) HYDROGENATED POLYBUTENE (1300 MW) (UNII: 7D1YQ9Y5EZ) PETROLATUM (UNII: 4T6H12BN9U) POLYBUTENE (1400 MW) (UNII: 1NA5AO9GH7) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) LANOLIN ALCOHOLS (UNII: 884C3FA9HE) CERESIN (UNII: Q1LS2UJO3A) MYRISTYL MYRISTATE (UNII: 4042ZC00DY) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CETYL LACTATE (UNII: A7EVH2RK4O) SHEA BUTTER (UNII: K49155WL9Y) CANDELILLA WAX (UNII: WL0328HX19) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) ZINC (UNII: J41CSQ7QDS) CARNAUBA WAX (UNII: R12CBM0EIZ) ETHYLHEXYL PALMITATE (UNII: 2865993309) TRIBEHENIN (UNII: 8OC9U7TQZ0) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) PALMITOYL TRIPEPTIDE-1 (UNII: RV743D216M) PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) STEARIC ACID (UNII: 4ELV7Z65AP) ISOPROPYL TITANIUM TRIISOSTEARATE (UNII: 949E3KBJ1I) COPPER (UNII: 789U1901C5) MICA (UNII: V8A1AW0880) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0255-1 1 in 1 BLISTER PACK 11/01/2010 1 4.2 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 11/01/2010 NEUTROGENA REVITALIZING LIP BALM SPF 20 - PETAL GLOW 40
octinoxate and oxybenzone lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0254 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 75 mg in 1 g OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 50 mg in 1 g Inactive Ingredients Ingredient Name Strength HYDROGENATED POLYBUTENE (1300 MW) (UNII: 7D1YQ9Y5EZ) PETROLATUM (UNII: 4T6H12BN9U) POLYBUTENE (1400 MW) (UNII: 1NA5AO9GH7) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) LANOLIN ALCOHOLS (UNII: 884C3FA9HE) CERESIN (UNII: Q1LS2UJO3A) MYRISTYL MYRISTATE (UNII: 4042ZC00DY) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CETYL LACTATE (UNII: A7EVH2RK4O) SHEA BUTTER (UNII: K49155WL9Y) CANDELILLA WAX (UNII: WL0328HX19) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) ZINC (UNII: J41CSQ7QDS) CARNAUBA WAX (UNII: R12CBM0EIZ) ETHYLHEXYL PALMITATE (UNII: 2865993309) TRIBEHENIN (UNII: 8OC9U7TQZ0) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) PALMITOYL TRIPEPTIDE-1 (UNII: RV743D216M) PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) ISOPROPYL TITANIUM TRIISOSTEARATE (UNII: 949E3KBJ1I) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) STEARIC ACID (UNII: 4ELV7Z65AP) COPPER (UNII: 789U1901C5) MICA (UNII: V8A1AW0880) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 7 (UNII: ECW0LZ41X8) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0254-1 1 in 1 BLISTER PACK 11/01/2010 1 4.2 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 11/01/2010 Labeler - Kenvue Brands LLC (118772437)