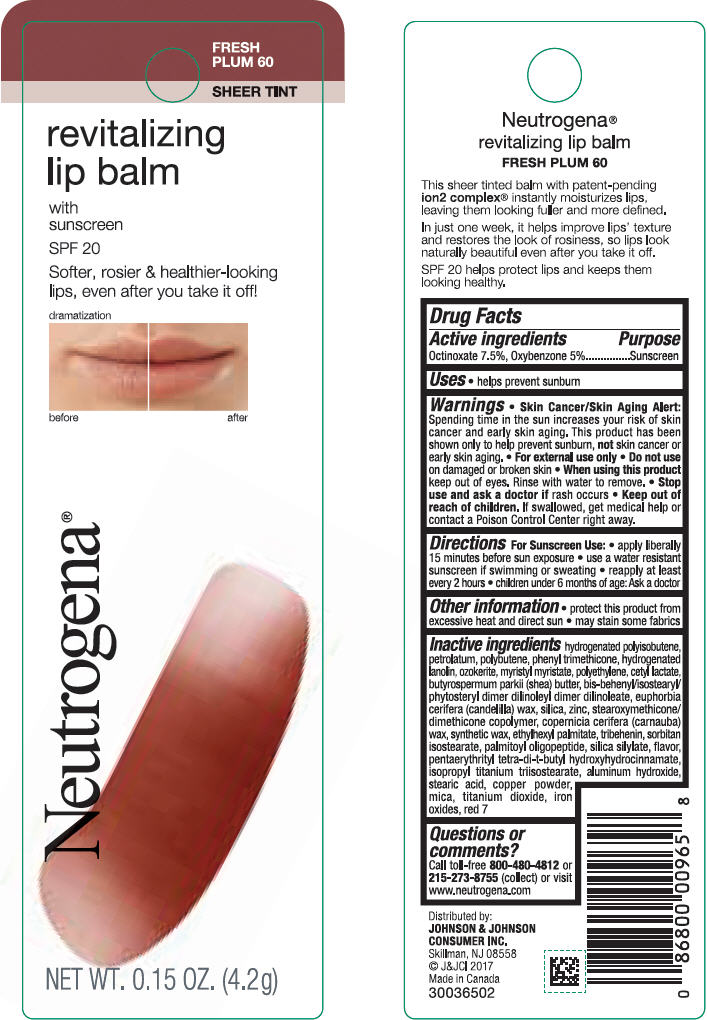
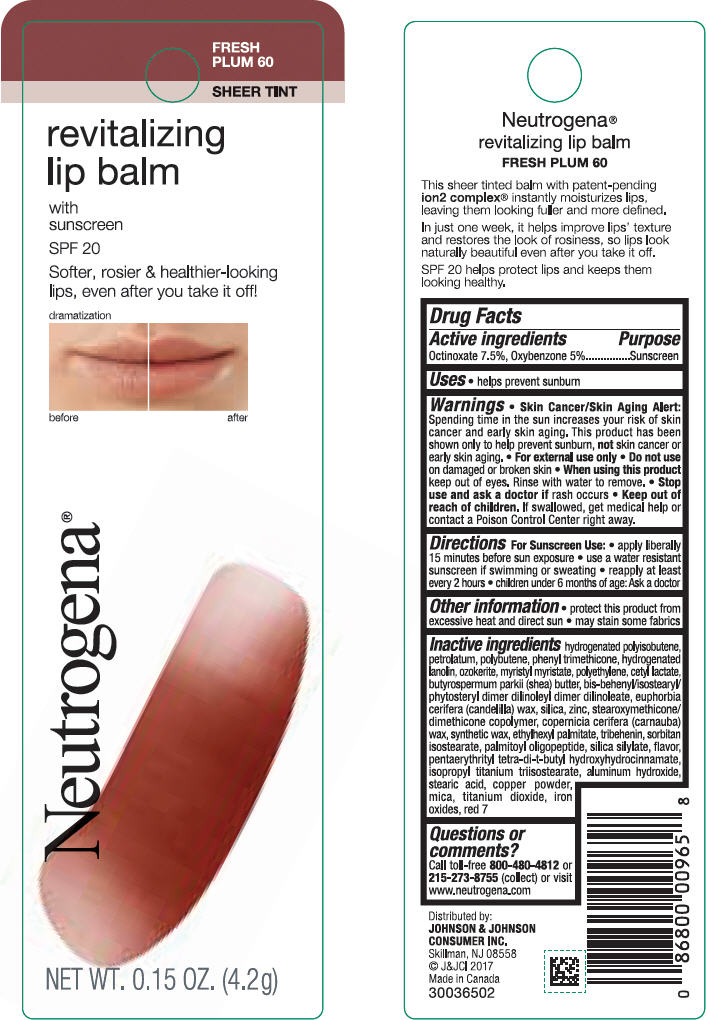
Label: NEUTROGENA REVITALIZING LIP BALM SPF 20 - FRESH PLUM 60- octinoxate and oxybenzone lipstick
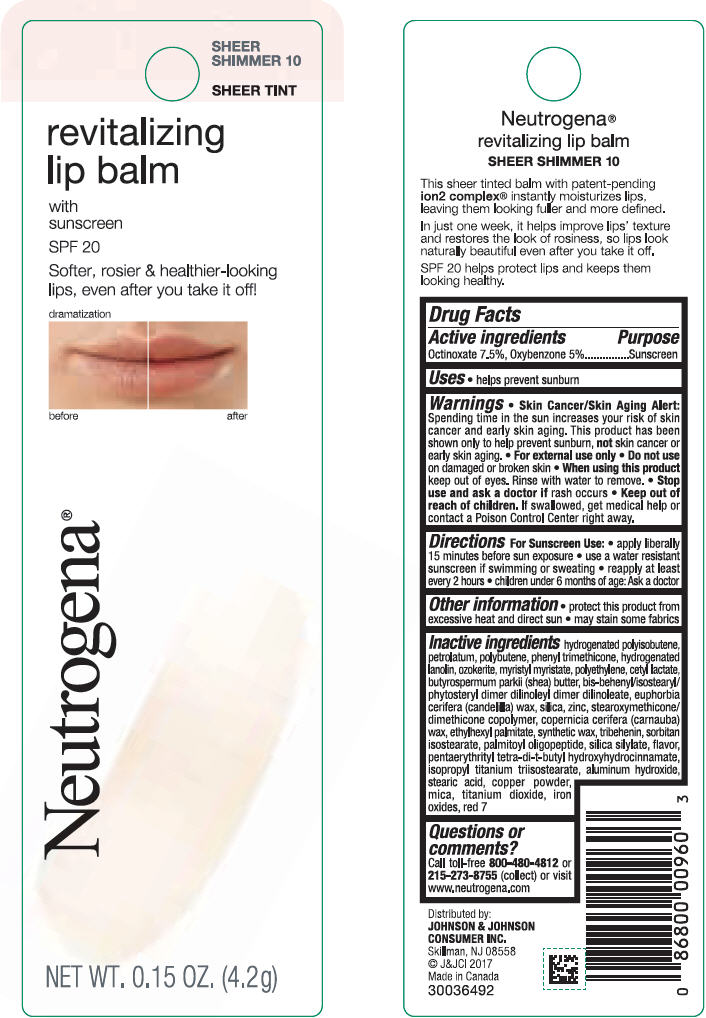
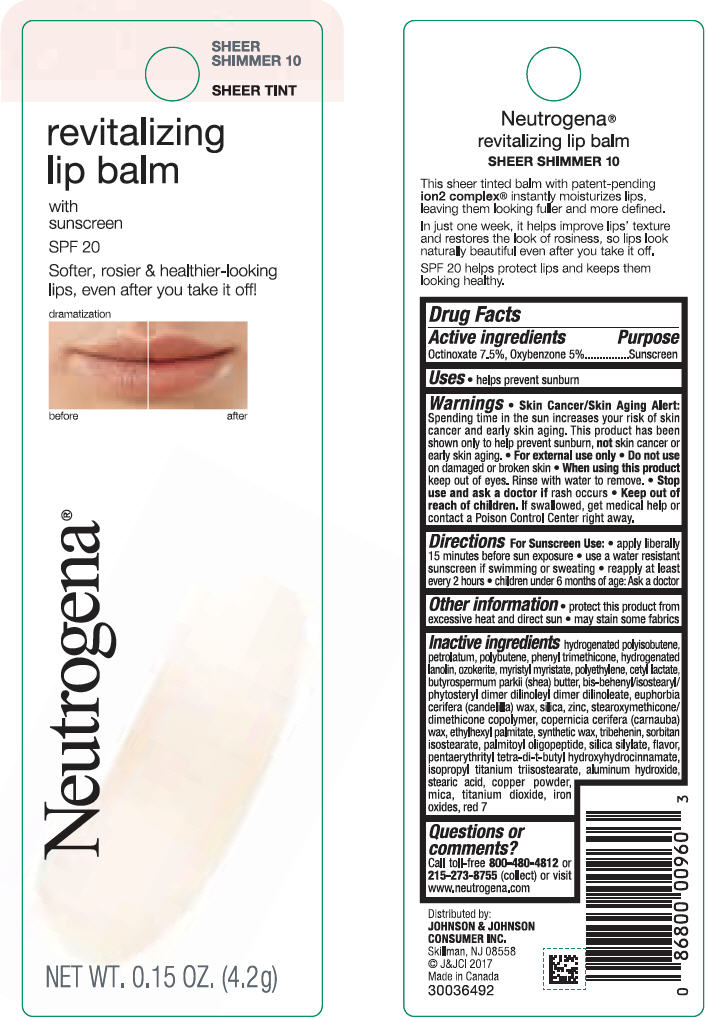
NEUTROGENA REVITALIZING LIP BALM SPF 20 - SHEEER SHIMMER 10- octinoxate and oxybenzone lipstick
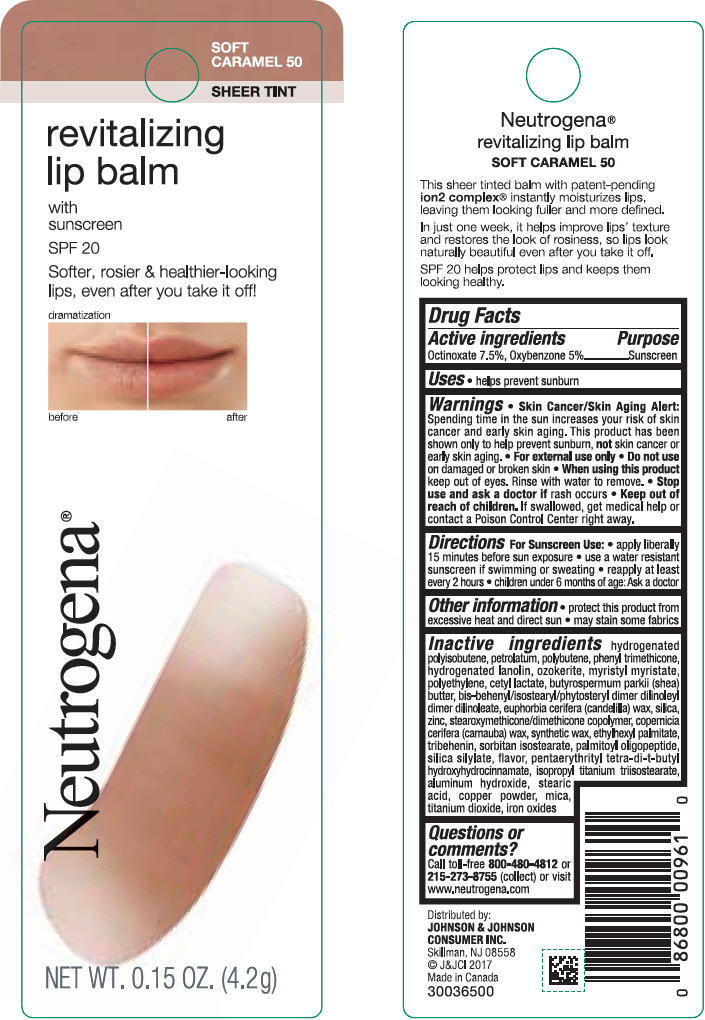
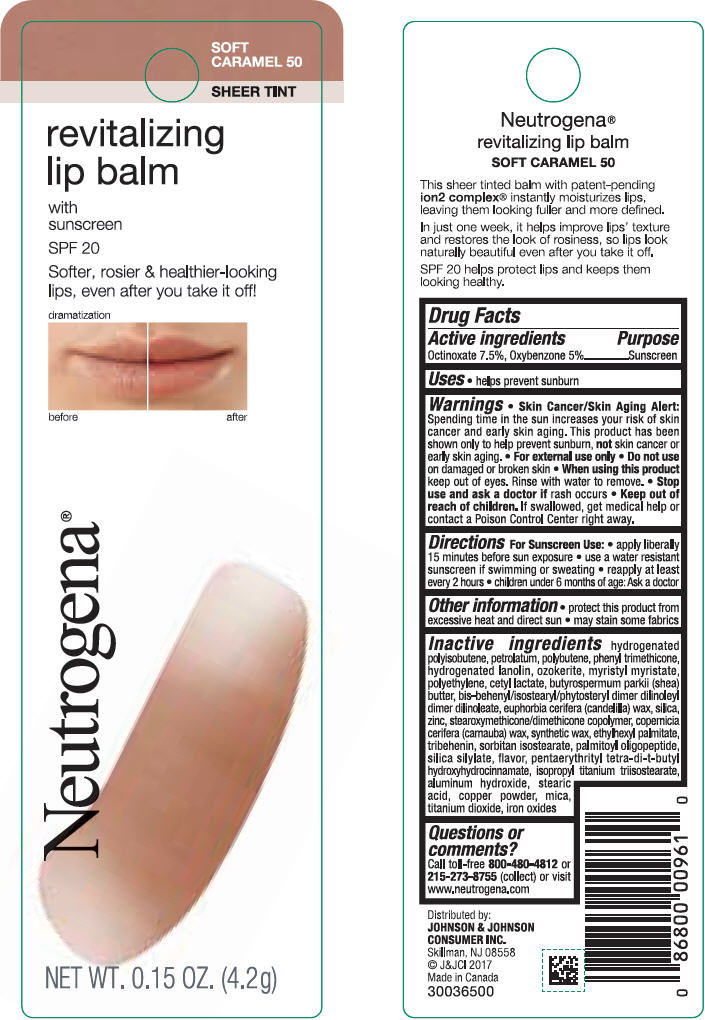
NEUTROGENA REVITALIZING LIP BALM SPF 20 - SOFT CARAMEL 50- octinoxate and oxybenzone lipstick
NEUTROGENA REVITALIZING LIP BALM SPF 20 - HEALTHY BLUSH 20- octinoxate and oxybenzone lipstick
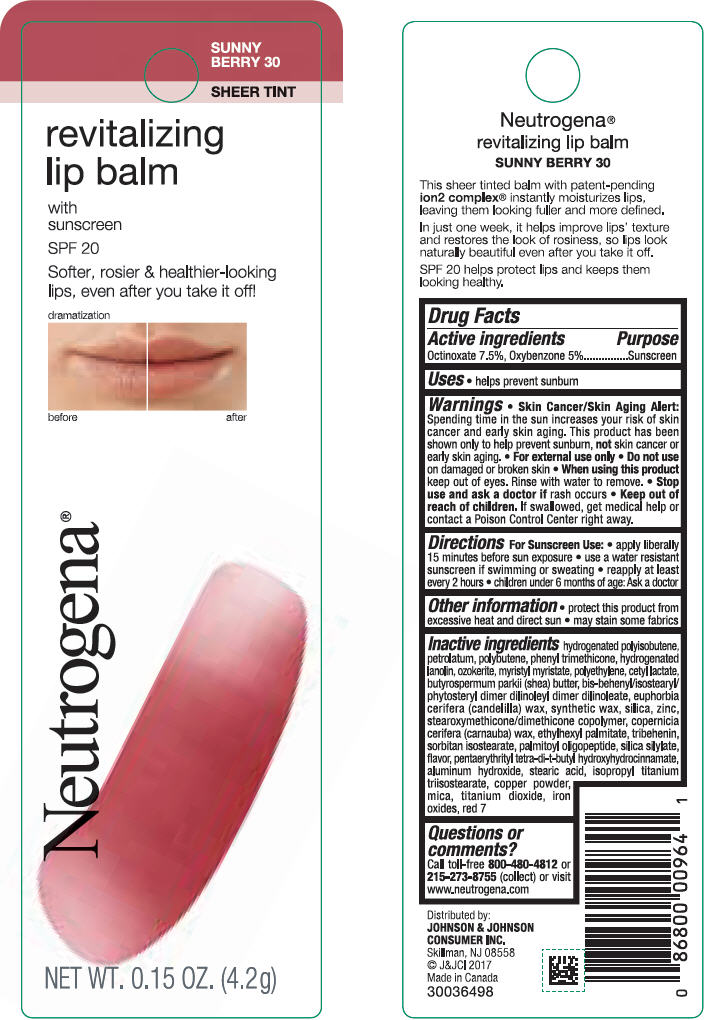
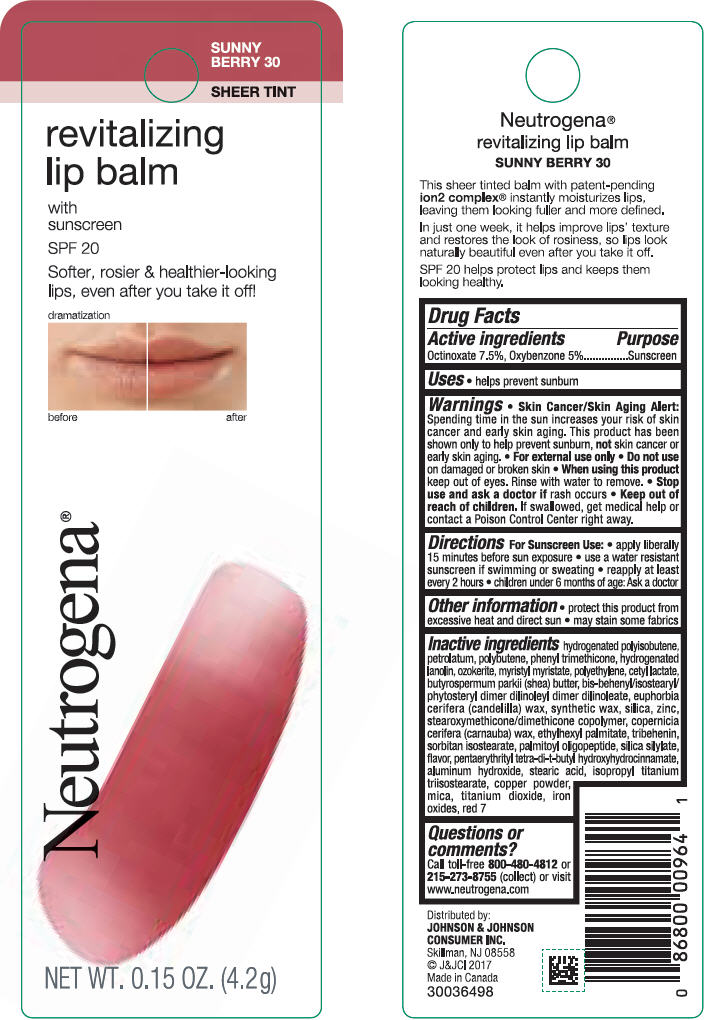
NEUTROGENA REVITALIZING LIP BALM SPF 20 - SUNNY BERRY 30- octinoxate and oxybenzone lipstick
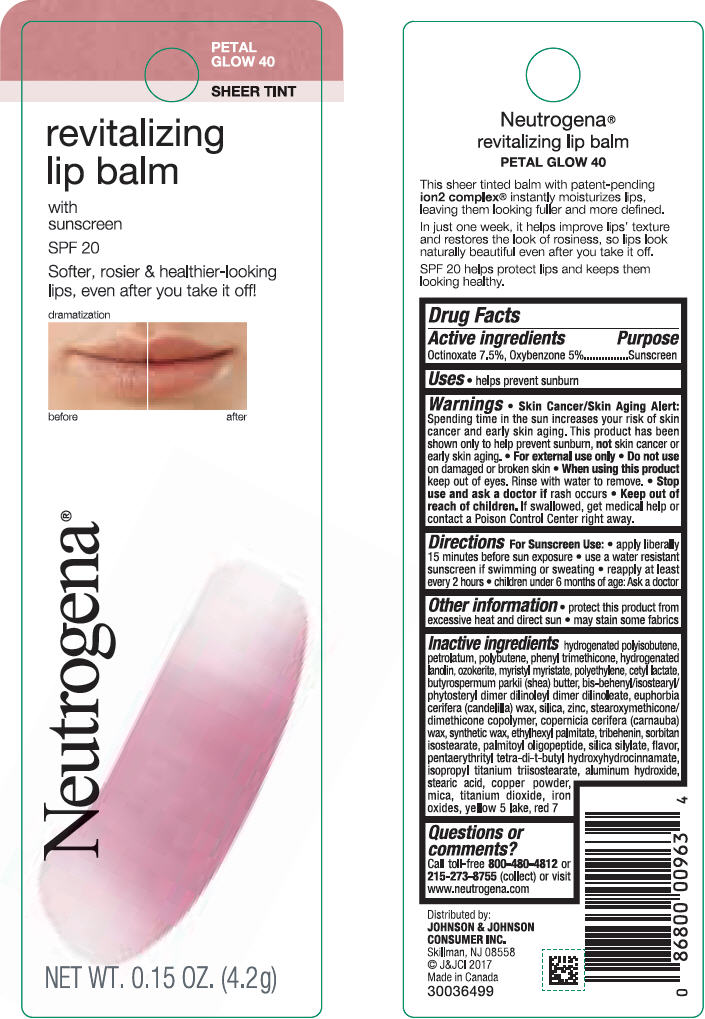
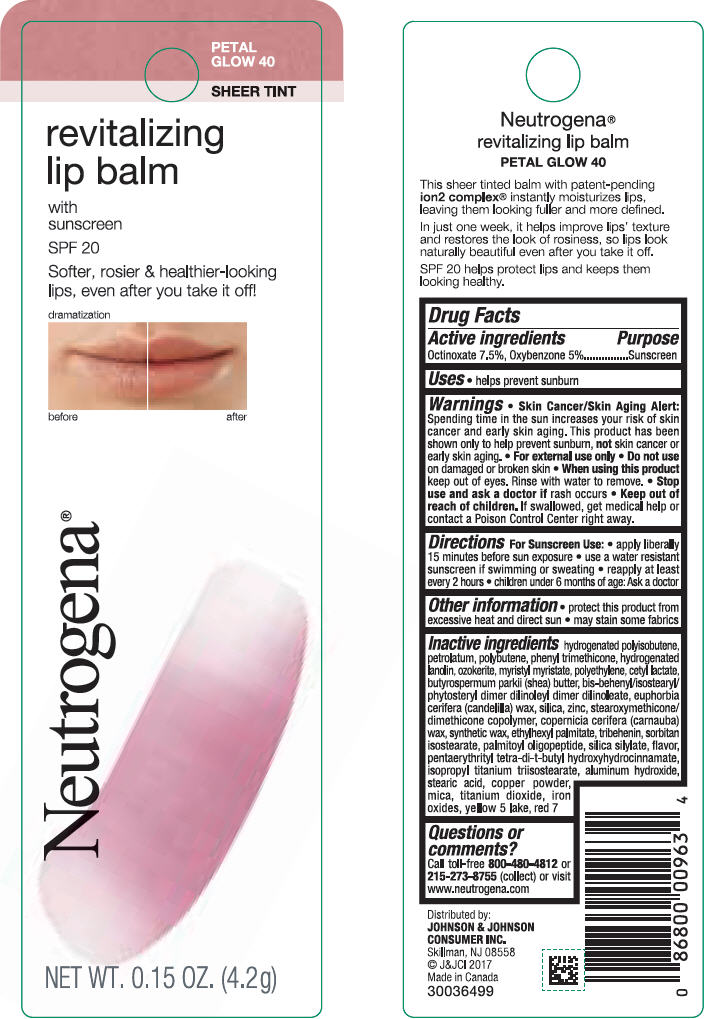
NEUTROGENA REVITALIZING LIP BALM SPF 20 - PETAL GLOW 40- octinoxate and oxybenzone lipstick
-
NDC Code(s):
69968-0251-1,
69968-0252-1,
69968-0253-1,
69968-0254-1, view more69968-0255-1, 69968-0256-1
- Packager: Johnson & Johnson Consumer Inc.
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 5, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredients
- Purpose
- Uses
- Warnings
- Directions
- Other information
-
Inactive ingredients
hydrogenated polyisobutene, petrolatum, polybutene, phenyl trimethicone, hydrogenated lanolin, ozokerite, myristyl myristate, polyethylene, cetyl lactate, butyrospermum parkii (shea) butter, bis-behenyl/isostearyl/phytosteryl dimer dilinoleyl dimer dilinoleate, euphorbia cerifera (candelilla) wax, silica, zinc, stearoxymethicone/dimethicone copolymer, copernicia cerifera (carnauba) wax, synthetic wax, ethylhexyl palmitate, tribehenin, sorbitan isostearate, palmitoyl oligopeptide, silica silylate, flavor, pentaerythrityl tetra-di-t-butyl hydroxyhydrocinnamate, isopropyl titanium triisostearate, aluminum hydroxide, stearic acid, copper powder, mica, titanium dioxide, iron oxides, red 7
- Questions or comments?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 4.2 g Tube Blister Pack - Fresh Plum 60
- PRINCIPAL DISPLAY PANEL - 4.2 g Tube Blister Pack - Sheer Shimmer 10
- PRINCIPAL DISPLAY PANEL - 4.2 g Tube Blister Pack - Soft Caramel 50
- PRINCIPAL DISPLAY PANEL - 4.2 g Tube Blister Pack - Healthy Blush 20
- PRINCIPAL DISPLAY PANEL - 4.2 g Tube Blister Pack - Sunny Berry 30
- PRINCIPAL DISPLAY PANEL - 4.2 g Tube Blister Pack - Petal Glow 40
-
INGREDIENTS AND APPEARANCE
NEUTROGENA REVITALIZING LIP BALM SPF 20 - FRESH PLUM 60
octinoxate and oxybenzone lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0256 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 75 mg in 1 g OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 50 mg in 1 g Inactive Ingredients Ingredient Name Strength HYDROGENATED POLYBUTENE (1300 MW) (UNII: 7D1YQ9Y5EZ) PETROLATUM (UNII: 4T6H12BN9U) POLYBUTENE (1400 MW) (UNII: 1NA5AO9GH7) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) LANOLIN ALCOHOLS (UNII: 884C3FA9HE) CERESIN (UNII: Q1LS2UJO3A) MYRISTYL MYRISTATE (UNII: 4042ZC00DY) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CETYL LACTATE (UNII: A7EVH2RK4O) SHEA BUTTER (UNII: K49155WL9Y) CANDELILLA WAX (UNII: WL0328HX19) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) ZINC (UNII: J41CSQ7QDS) CARNAUBA WAX (UNII: R12CBM0EIZ) ETHYLHEXYL PALMITATE (UNII: 2865993309) TRIBEHENIN (UNII: 8OC9U7TQZ0) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) PALMITOYL TRIPEPTIDE-1 (UNII: RV743D216M) PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) ISOPROPYL TITANIUM TRIISOSTEARATE (UNII: 949E3KBJ1I) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) STEARIC ACID (UNII: 4ELV7Z65AP) COPPER (UNII: 789U1901C5) MICA (UNII: V8A1AW0880) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) D&C RED NO. 7 (UNII: ECW0LZ41X8) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0256-1 1 in 1 BLISTER PACK 11/01/2010 1 4.2 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/01/2010 NEUTROGENA REVITALIZING LIP BALM SPF 20 - SHEEER SHIMMER 10
octinoxate and oxybenzone lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0251 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 75 mg in 1 g OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 50 mg in 1 g Inactive Ingredients Ingredient Name Strength HYDROGENATED POLYBUTENE (1300 MW) (UNII: 7D1YQ9Y5EZ) PETROLATUM (UNII: 4T6H12BN9U) POLYBUTENE (1400 MW) (UNII: 1NA5AO9GH7) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) LANOLIN ALCOHOLS (UNII: 884C3FA9HE) CERESIN (UNII: Q1LS2UJO3A) MYRISTYL MYRISTATE (UNII: 4042ZC00DY) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CETYL LACTATE (UNII: A7EVH2RK4O) SHEA BUTTER (UNII: K49155WL9Y) CANDELILLA WAX (UNII: WL0328HX19) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) ZINC (UNII: J41CSQ7QDS) CARNAUBA WAX (UNII: R12CBM0EIZ) ETHYLHEXYL PALMITATE (UNII: 2865993309) TRIBEHENIN (UNII: 8OC9U7TQZ0) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) PALMITOYL TRIPEPTIDE-1 (UNII: RV743D216M) PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) ISOPROPYL TITANIUM TRIISOSTEARATE (UNII: 949E3KBJ1I) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) STEARIC ACID (UNII: 4ELV7Z65AP) COPPER (UNII: 789U1901C5) MICA (UNII: V8A1AW0880) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) D&C RED NO. 7 (UNII: ECW0LZ41X8) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0251-1 1 in 1 BLISTER PACK 11/01/2010 1 4.2 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/01/2010 NEUTROGENA REVITALIZING LIP BALM SPF 20 - SOFT CARAMEL 50
octinoxate and oxybenzone lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0252 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 75 mg in 1 g OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 50 mg in 1 g Inactive Ingredients Ingredient Name Strength HYDROGENATED POLYBUTENE (1300 MW) (UNII: 7D1YQ9Y5EZ) PETROLATUM (UNII: 4T6H12BN9U) POLYBUTENE (1400 MW) (UNII: 1NA5AO9GH7) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) LANOLIN ALCOHOLS (UNII: 884C3FA9HE) CERESIN (UNII: Q1LS2UJO3A) MYRISTYL MYRISTATE (UNII: 4042ZC00DY) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CETYL LACTATE (UNII: A7EVH2RK4O) SHEA BUTTER (UNII: K49155WL9Y) CANDELILLA WAX (UNII: WL0328HX19) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) ZINC (UNII: J41CSQ7QDS) CARNAUBA WAX (UNII: R12CBM0EIZ) ETHYLHEXYL PALMITATE (UNII: 2865993309) TRIBEHENIN (UNII: 8OC9U7TQZ0) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) PALMITOYL TRIPEPTIDE-1 (UNII: RV743D216M) PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) ISOPROPYL TITANIUM TRIISOSTEARATE (UNII: 949E3KBJ1I) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) STEARIC ACID (UNII: 4ELV7Z65AP) COPPER (UNII: 789U1901C5) MICA (UNII: V8A1AW0880) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0252-1 1 in 1 BLISTER PACK 11/01/2010 1 4.2 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/01/2010 NEUTROGENA REVITALIZING LIP BALM SPF 20 - HEALTHY BLUSH 20
octinoxate and oxybenzone lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0253 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 75 mg in 1 g OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 50 mg in 1 g Inactive Ingredients Ingredient Name Strength HYDROGENATED POLYBUTENE (1300 MW) (UNII: 7D1YQ9Y5EZ) PETROLATUM (UNII: 4T6H12BN9U) POLYBUTENE (1400 MW) (UNII: 1NA5AO9GH7) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) LANOLIN ALCOHOLS (UNII: 884C3FA9HE) CERESIN (UNII: Q1LS2UJO3A) MYRISTYL MYRISTATE (UNII: 4042ZC00DY) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CETYL LACTATE (UNII: A7EVH2RK4O) SHEA BUTTER (UNII: K49155WL9Y) CANDELILLA WAX (UNII: WL0328HX19) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) ZINC (UNII: J41CSQ7QDS) CARNAUBA WAX (UNII: R12CBM0EIZ) ETHYLHEXYL PALMITATE (UNII: 2865993309) TRIBEHENIN (UNII: 8OC9U7TQZ0) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) PALMITOYL TRIPEPTIDE-1 (UNII: RV743D216M) PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) ISOPROPYL TITANIUM TRIISOSTEARATE (UNII: 949E3KBJ1I) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) STEARIC ACID (UNII: 4ELV7Z65AP) COPPER (UNII: 789U1901C5) MICA (UNII: V8A1AW0880) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 6 (UNII: 481744AI4O) D&C RED NO. 7 (UNII: ECW0LZ41X8) STANNIC OXIDE (UNII: KM7N50LOS6) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0253-1 1 in 1 BLISTER PACK 11/01/2010 1 4.2 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/01/2010 NEUTROGENA REVITALIZING LIP BALM SPF 20 - SUNNY BERRY 30
octinoxate and oxybenzone lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0255 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 75 mg in 1 g OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 50 mg in 1 g Inactive Ingredients Ingredient Name Strength HYDROGENATED POLYBUTENE (1300 MW) (UNII: 7D1YQ9Y5EZ) PETROLATUM (UNII: 4T6H12BN9U) POLYBUTENE (1400 MW) (UNII: 1NA5AO9GH7) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) LANOLIN ALCOHOLS (UNII: 884C3FA9HE) CERESIN (UNII: Q1LS2UJO3A) MYRISTYL MYRISTATE (UNII: 4042ZC00DY) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CETYL LACTATE (UNII: A7EVH2RK4O) SHEA BUTTER (UNII: K49155WL9Y) CANDELILLA WAX (UNII: WL0328HX19) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) ZINC (UNII: J41CSQ7QDS) CARNAUBA WAX (UNII: R12CBM0EIZ) ETHYLHEXYL PALMITATE (UNII: 2865993309) TRIBEHENIN (UNII: 8OC9U7TQZ0) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) PALMITOYL TRIPEPTIDE-1 (UNII: RV743D216M) PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) STEARIC ACID (UNII: 4ELV7Z65AP) ISOPROPYL TITANIUM TRIISOSTEARATE (UNII: 949E3KBJ1I) COPPER (UNII: 789U1901C5) MICA (UNII: V8A1AW0880) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) D&C RED NO. 7 (UNII: ECW0LZ41X8) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0255-1 1 in 1 BLISTER PACK 11/01/2010 1 4.2 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/01/2010 NEUTROGENA REVITALIZING LIP BALM SPF 20 - PETAL GLOW 40
octinoxate and oxybenzone lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0254 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 75 mg in 1 g OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 50 mg in 1 g Inactive Ingredients Ingredient Name Strength HYDROGENATED POLYBUTENE (1300 MW) (UNII: 7D1YQ9Y5EZ) PETROLATUM (UNII: 4T6H12BN9U) POLYBUTENE (1400 MW) (UNII: 1NA5AO9GH7) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) LANOLIN ALCOHOLS (UNII: 884C3FA9HE) CERESIN (UNII: Q1LS2UJO3A) MYRISTYL MYRISTATE (UNII: 4042ZC00DY) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CETYL LACTATE (UNII: A7EVH2RK4O) SHEA BUTTER (UNII: K49155WL9Y) CANDELILLA WAX (UNII: WL0328HX19) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) ZINC (UNII: J41CSQ7QDS) CARNAUBA WAX (UNII: R12CBM0EIZ) ETHYLHEXYL PALMITATE (UNII: 2865993309) TRIBEHENIN (UNII: 8OC9U7TQZ0) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) PALMITOYL TRIPEPTIDE-1 (UNII: RV743D216M) PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) ISOPROPYL TITANIUM TRIISOSTEARATE (UNII: 949E3KBJ1I) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) STEARIC ACID (UNII: 4ELV7Z65AP) COPPER (UNII: 789U1901C5) MICA (UNII: V8A1AW0880) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 7 (UNII: ECW0LZ41X8) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0254-1 1 in 1 BLISTER PACK 11/01/2010 1 4.2 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/01/2010 Labeler - Johnson & Johnson Consumer Inc. (118772437)