Label: PRAMIPEXOLE DIHYDROCHLORIDE tablet, extended release
-
NDC Code(s):
70771-1328-2,
70771-1328-3,
70771-1328-4,
70771-1329-2, view more70771-1329-3, 70771-1329-4, 70771-1330-2, 70771-1330-3, 70771-1330-4, 70771-1331-3, 70771-1332-2, 70771-1332-3, 70771-1332-4, 70771-1333-2, 70771-1333-3, 70771-1333-4, 70771-1334-2, 70771-1334-3, 70771-1334-4
- Packager: Zydus Lifesciences Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated October 13, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Pramipexole Dihydrochloride Extended-release Tablets, 0.375 mg
Rx only
30 tablets

Pramipexole Dihydrochloride Extended-release Tablets, 0.75 mg
Rx only
30 tablets

Pramipexole Dihydrochloride Extended-release Tablets, 1.5 mg
Rx only
30 tablets

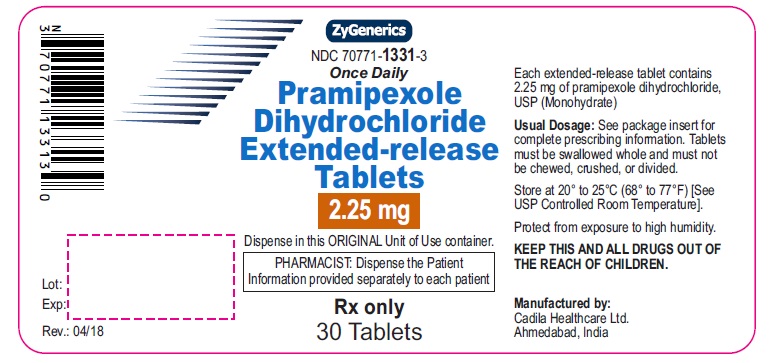
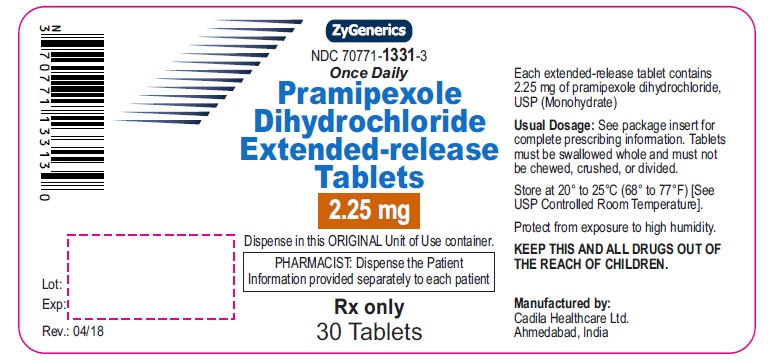
Pramipexole Dihydrochloride Extended-release Tablets, 2.25 mg
Rx only
30 tablets
Pramipexole Dihydrochloride Extended-release Tablets, 3 mg
Rx only
30 tablets

Pramipexole Dihydrochloride Extended-release Tablets, 3.75 mg
Rx only
30 tablets

Pramipexole Dihydrochloride Extended-release Tablets, 4.5 mg
Rx only
30 tablets

-
INGREDIENTS AND APPEARANCE
PRAMIPEXOLE DIHYDROCHLORIDE
pramipexole dihydrochloride tablet, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:70771-1328 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PRAMIPEXOLE DIHYDROCHLORIDE (UNII: 3D867NP06J) (PRAMIPEXOLE - UNII:83619PEU5T) PRAMIPEXOLE DIHYDROCHLORIDE 0.375 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) DIBASIC CALCIUM PHOSPHATE DIHYDRATE (UNII: O7TSZ97GEP) HYDROGENATED CASTOR OIL (UNII: ZF94AP8MEY) ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) MANNITOL (UNII: 3OWL53L36A) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) Product Characteristics Color WHITE (WHITE TO OFF-WHITE) , BROWN (Buff to light brown speckles) Score no score Shape OVAL (oval) Size 11mm Flavor Imprint Code 474 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70771-1328-3 30 in 1 BOTTLE; Type 0: Not a Combination Product 04/24/2018 2 NDC:70771-1328-4 10 in 1 CARTON 04/24/2018 2 NDC:70771-1328-2 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA202891 04/24/2018 PRAMIPEXOLE DIHYDROCHLORIDE
pramipexole dihydrochloride tablet, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:70771-1329 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PRAMIPEXOLE DIHYDROCHLORIDE (UNII: 3D867NP06J) (PRAMIPEXOLE - UNII:83619PEU5T) PRAMIPEXOLE DIHYDROCHLORIDE 0.75 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) DIBASIC CALCIUM PHOSPHATE DIHYDRATE (UNII: O7TSZ97GEP) HYDROGENATED CASTOR OIL (UNII: ZF94AP8MEY) ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) MANNITOL (UNII: 3OWL53L36A) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) Product Characteristics Color WHITE (WHITE TO OFF-WHITE) , BROWN (Buff to light brown speckles) Score no score Shape OVAL (oval) Size 11mm Flavor Imprint Code 475 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70771-1329-3 30 in 1 BOTTLE; Type 0: Not a Combination Product 04/24/2018 2 NDC:70771-1329-4 10 in 1 CARTON 04/24/2018 2 NDC:70771-1329-2 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA202891 04/24/2018 PRAMIPEXOLE DIHYDROCHLORIDE
pramipexole dihydrochloride tablet, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:70771-1330 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PRAMIPEXOLE DIHYDROCHLORIDE (UNII: 3D867NP06J) (PRAMIPEXOLE - UNII:83619PEU5T) PRAMIPEXOLE DIHYDROCHLORIDE 1.5 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) DIBASIC CALCIUM PHOSPHATE DIHYDRATE (UNII: O7TSZ97GEP) HYDROGENATED CASTOR OIL (UNII: ZF94AP8MEY) ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) MANNITOL (UNII: 3OWL53L36A) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) Product Characteristics Color WHITE (WHITE TO OFF-WHITE) , BROWN (Buff to light brown speckles) Score no score Shape OVAL (oval) Size 13mm Flavor Imprint Code 476 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70771-1330-3 30 in 1 BOTTLE; Type 0: Not a Combination Product 04/24/2018 2 NDC:70771-1330-4 10 in 1 CARTON 04/24/2018 2 NDC:70771-1330-2 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA202891 04/24/2018 PRAMIPEXOLE DIHYDROCHLORIDE
pramipexole dihydrochloride tablet, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:70771-1332 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PRAMIPEXOLE DIHYDROCHLORIDE (UNII: 3D867NP06J) (PRAMIPEXOLE - UNII:83619PEU5T) PRAMIPEXOLE DIHYDROCHLORIDE 3 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) DIBASIC CALCIUM PHOSPHATE DIHYDRATE (UNII: O7TSZ97GEP) HYDROGENATED CASTOR OIL (UNII: ZF94AP8MEY) ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) MANNITOL (UNII: 3OWL53L36A) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) Product Characteristics Color WHITE (WHITE TO OFF-WHITE) , BROWN (Buff to light brown speckles) Score no score Shape OVAL (oval) Size 13mm Flavor Imprint Code 477 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70771-1332-3 30 in 1 BOTTLE; Type 0: Not a Combination Product 04/24/2018 2 NDC:70771-1332-4 10 in 1 CARTON 04/24/2018 2 NDC:70771-1332-2 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA202891 04/24/2018 PRAMIPEXOLE DIHYDROCHLORIDE
pramipexole dihydrochloride tablet, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:70771-1334 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PRAMIPEXOLE DIHYDROCHLORIDE (UNII: 3D867NP06J) (PRAMIPEXOLE - UNII:83619PEU5T) PRAMIPEXOLE DIHYDROCHLORIDE 4.5 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) DIBASIC CALCIUM PHOSPHATE DIHYDRATE (UNII: O7TSZ97GEP) HYDROGENATED CASTOR OIL (UNII: ZF94AP8MEY) ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) MANNITOL (UNII: 3OWL53L36A) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) Product Characteristics Color WHITE (WHITE TO OFF-WHITE) , BROWN (Buff to light brown speckles) Score no score Shape OVAL (oval) Size 16mm Flavor Imprint Code 478 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70771-1334-3 30 in 1 BOTTLE; Type 0: Not a Combination Product 04/24/2018 2 NDC:70771-1334-4 10 in 1 CARTON 04/24/2018 2 NDC:70771-1334-2 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA202891 04/24/2018 PRAMIPEXOLE DIHYDROCHLORIDE
pramipexole dihydrochloride tablet, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:70771-1331 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PRAMIPEXOLE DIHYDROCHLORIDE (UNII: 3D867NP06J) (PRAMIPEXOLE - UNII:83619PEU5T) PRAMIPEXOLE DIHYDROCHLORIDE 2.25 mg Inactive Ingredients Ingredient Name Strength ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) DIBASIC CALCIUM PHOSPHATE DIHYDRATE (UNII: O7TSZ97GEP) HYDROGENATED CASTOR OIL (UNII: ZF94AP8MEY) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) MANNITOL (UNII: 3OWL53L36A) STARCH, CORN (UNII: O8232NY3SJ) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) Product Characteristics Color WHITE (OFF-WHITE) Score no score Shape OVAL (OVAL) Size 13mm Flavor Imprint Code 874 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70771-1331-3 30 in 1 BOTTLE; Type 0: Not a Combination Product 04/24/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA202891 04/24/2018 PRAMIPEXOLE DIHYDROCHLORIDE
pramipexole dihydrochloride tablet, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:70771-1333 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PRAMIPEXOLE DIHYDROCHLORIDE (UNII: 3D867NP06J) (PRAMIPEXOLE - UNII:83619PEU5T) PRAMIPEXOLE DIHYDROCHLORIDE 3.75 mg Inactive Ingredients Ingredient Name Strength ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) DIBASIC CALCIUM PHOSPHATE DIHYDRATE (UNII: O7TSZ97GEP) HYDROGENATED CASTOR OIL (UNII: ZF94AP8MEY) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) MANNITOL (UNII: 3OWL53L36A) STARCH, CORN (UNII: O8232NY3SJ) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) Product Characteristics Color WHITE (OFF-WHITE) Score no score Shape OVAL (OVAL) Size 13mm Flavor Imprint Code 875 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70771-1333-3 30 in 1 BOTTLE; Type 0: Not a Combination Product 04/24/2018 2 NDC:70771-1333-4 10 in 1 CARTON 04/24/2018 2 NDC:70771-1333-2 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA202891 04/24/2018 Labeler - Zydus Lifesciences Limited (918596198) Registrant - Zydus Lifesciences Limited (918596198) Establishment Name Address ID/FEI Business Operations Zydus Lifesciences Limited 918596198 ANALYSIS(70771-1328, 70771-1329, 70771-1330, 70771-1331, 70771-1332, 70771-1333, 70771-1334) , MANUFACTURE(70771-1328, 70771-1329, 70771-1330, 70771-1331, 70771-1332, 70771-1333, 70771-1334)