PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Pramipexole Dihydrochloride Extended-release Tablets, 0.375 mg
Rx only
30 tablets

Pramipexole Dihydrochloride Extended-release Tablets, 0.75 mg
Rx only
30 tablets

Pramipexole Dihydrochloride Extended-release Tablets, 1.5 mg
Rx only
30 tablets

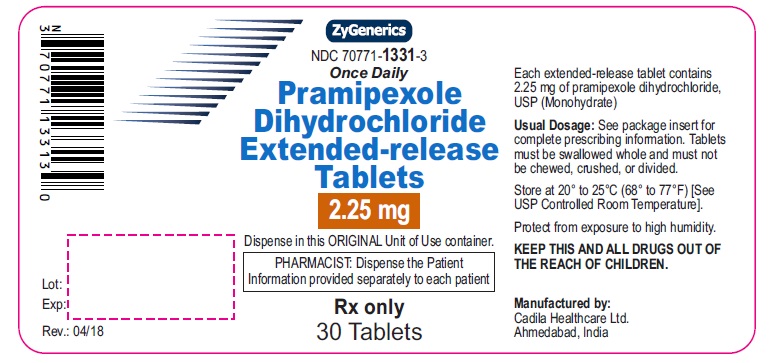
Pramipexole Dihydrochloride Extended-release Tablets, 2.25 mg
Rx only
30 tablets
Pramipexole Dihydrochloride Extended-release Tablets, 3 mg
Rx only
30 tablets

Pramipexole Dihydrochloride Extended-release Tablets, 3.75 mg
Rx only
30 tablets

Pramipexole Dihydrochloride Extended-release Tablets, 4.5 mg
Rx only
30 tablets
