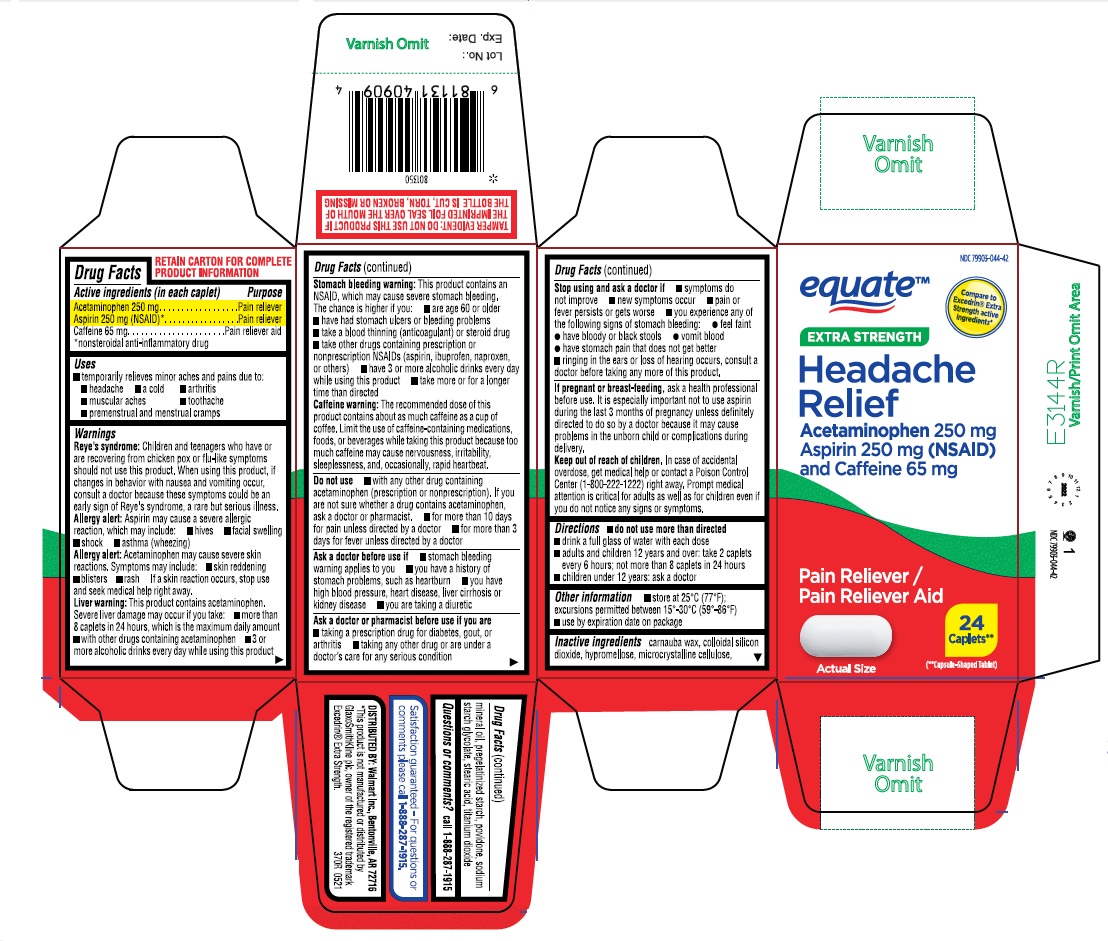
Label: EXTRA STRENGTH HEADACHE RELIEF- acetaminophen aspirin caffeine tablet, coated
- NDC Code(s): 79903-044-20, 79903-044-42
- Packager: WAL-MART STORES INC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated April 3, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- DRUG FACTS
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
WARNINGS
Warnings
Reye’s syndrome: Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye’s syndrome, a rare but serious illness.
Allergy alert: Aspirin may cause a severe allergic reaction, which may include:
• hives
• facial swelling
• shock• asthma (wheezing)
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
• skin reddening
• blisters
• rash
If a skin reaction occurs, stop use and seek medical help right away.
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take:
• more than 8 caplets in 24 hours, which is the maximum daily amount
• with other drugs containing acetaminophen
• 3 or more alcoholic drinks every day while using this productStomach bleeding warning: This product contains an NSAID, which may cause severe stomach bleeding. The chance is higher if you:
• are age 60 or older
• have had stomach ulcers or bleeding problems
• take a blood thinning (anticoagulant) or steroid drug
• take other drugs containing prescription or nonprescription NSAIDs (aspirin, ibuprofen, naproxen, or others)
• have 3 or more alcoholic drinks every day while using this product
• take more or for a longer time than directed
Caffeine warning: The recommended dose of this product contains about as much caffeine as a cup of coffee. Limit the use of caffeine-containing medications, foods, or beverages while taking this product because too much caffeine may cause nervousness, irritability, sleeplessness, and, occasionally, rapid heartbeat. - DO NOT USE
- ASK DOCTOR
- ASK DOCTOR/PHARMACIST
-
STOP USE
Stop use and ask a doctor if
- symptoms do not improve
- new symptoms occur
- pain or fever persists or gets worse
- you experience any of the following signs of stomach bleeding:
feel faint
have bloody or black stools
vomit blood
have stomach pain that does not get better
- ringing in the ears or loss of hearing occurs, consult a doctor before taking any more of this product
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- OTHER SAFETY INFORMATION
- INACTIVE INGREDIENT
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
EXTRA STRENGTH HEADACHE RELIEF
acetaminophen aspirin caffeine tablet, coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:79903-044 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ASPIRIN (UNII: R16CO5Y76E) (ASPIRIN - UNII:R16CO5Y76E) ASPIRIN 250 mg ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 250 mg CAFFEINE (UNII: 3G6A5W338E) (CAFFEINE - UNII:3G6A5W338E) CAFFEINE 65 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POVIDONE (UNII: FZ989GH94E) STEARIC ACID (UNII: 4ELV7Z65AP) CARNAUBA WAX (UNII: R12CBM0EIZ) SODIUM STARCH GLYCOLATE TYPE A CORN (UNII: AG9B65PV6B) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) HYPROMELLOSES (UNII: 3NXW29V3WO) MINERAL OIL (UNII: T5L8T28FGP) STARCH, CORN (UNII: O8232NY3SJ) Product Characteristics Color white Score no score Shape CAPSULE (Capsule shaped tablet) Size 17mm Flavor Imprint Code TCL370 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:79903-044-20 200 in 1 BOTTLE; Type 0: Not a Combination Product 03/09/2022 2 NDC:79903-044-42 1 in 1 CARTON 03/09/2022 2 24 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M013 03/09/2022 Labeler - WAL-MART STORES INC (051957769) Registrant - TIME CAP LABORATORIES, INC. (037052099) Establishment Name Address ID/FEI Business Operations MARKSANS PHARMA LIMITED 925822975 manufacture(79903-044)