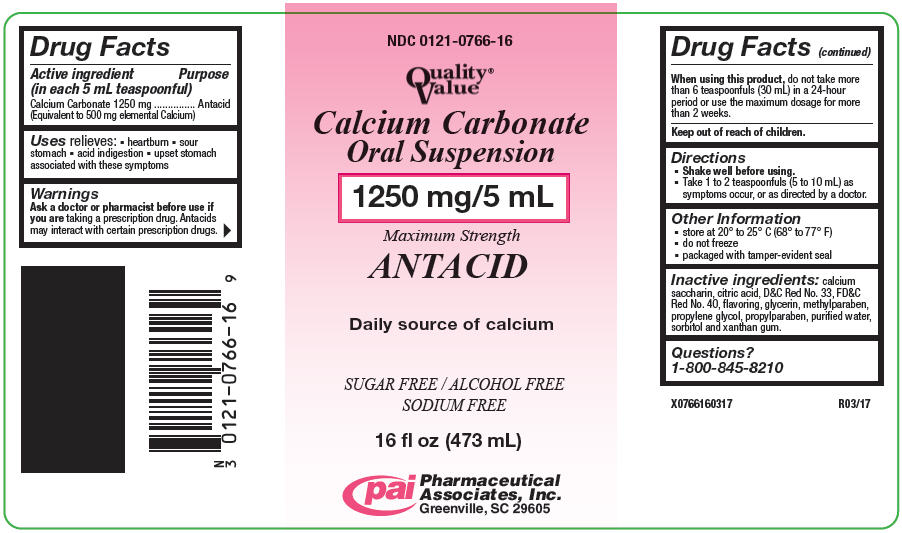
Label: CALCIUM CARBONATE suspension
- NDC Code(s): 0121-0766-16, 0121-4766-05
- Packager: PAI Holdings, LLC dba PAI Pharma
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient (in each 5 mL teaspoonful)
- Purpose
- Uses
- Warnings
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 473 mL Bottle Label
- PRINCIPAL DISPLAY PANEL - 5 mL Cup Tray Label
-
INGREDIENTS AND APPEARANCE
CALCIUM CARBONATE
calcium carbonate suspensionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0121-0766 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CALCIUM CARBONATE (UNII: H0G9379FGK) (CALCIUM CATION - UNII:2M83C4R6ZB, CARBONATE ION - UNII:7UJQ5OPE7D) CALCIUM CARBONATE 1250 mg in 5 mL Inactive Ingredients Ingredient Name Strength D&C RED NO. 33 (UNII: 9DBA0SBB0L) SACCHARIN CALCIUM (UNII: 5101OP7P2I) WATER (UNII: 059QF0KO0R) PROPYLPARABEN (UNII: Z8IX2SC1OH) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SORBITOL (UNII: 506T60A25R) GLYCERIN (UNII: PDC6A3C0OX) XANTHAN GUM (UNII: TTV12P4NEE) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) FD&C RED NO. 40 (UNII: WZB9127XOA) Product Characteristics Color pink Score Shape Size Flavor BUBBLE GUM Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0121-0766-16 12 in 1 CASE 12/01/2004 1 473 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M001 12/01/2004 CALCIUM CARBONATE
calcium carbonate suspensionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0121-4766 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CALCIUM CARBONATE (UNII: H0G9379FGK) (CALCIUM CATION - UNII:2M83C4R6ZB, CARBONATE ION - UNII:7UJQ5OPE7D) CALCIUM CARBONATE 1250 mg in 5 mL Inactive Ingredients Ingredient Name Strength PROPYLPARABEN (UNII: Z8IX2SC1OH) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SORBITOL (UNII: 506T60A25R) GLYCERIN (UNII: PDC6A3C0OX) XANTHAN GUM (UNII: TTV12P4NEE) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) FD&C RED NO. 40 (UNII: WZB9127XOA) D&C RED NO. 33 (UNII: 9DBA0SBB0L) SACCHARIN CALCIUM (UNII: 5101OP7P2I) WATER (UNII: 059QF0KO0R) Product Characteristics Color pink Score Shape Size Flavor BUBBLE GUM Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0121-4766-05 4 in 1 CASE 12/01/2004 1 10 in 1 TRAY 1 5 mL in 1 CUP, UNIT-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M001 12/01/2004 Labeler - PAI Holdings, LLC dba PAI Pharma (044940096) Establishment Name Address ID/FEI Business Operations PAI Holdings, LLC dba Pharmaceutical Associates, Inc. and dba PAI Pharma 097630693 manufacture(0121-0766, 0121-4766)