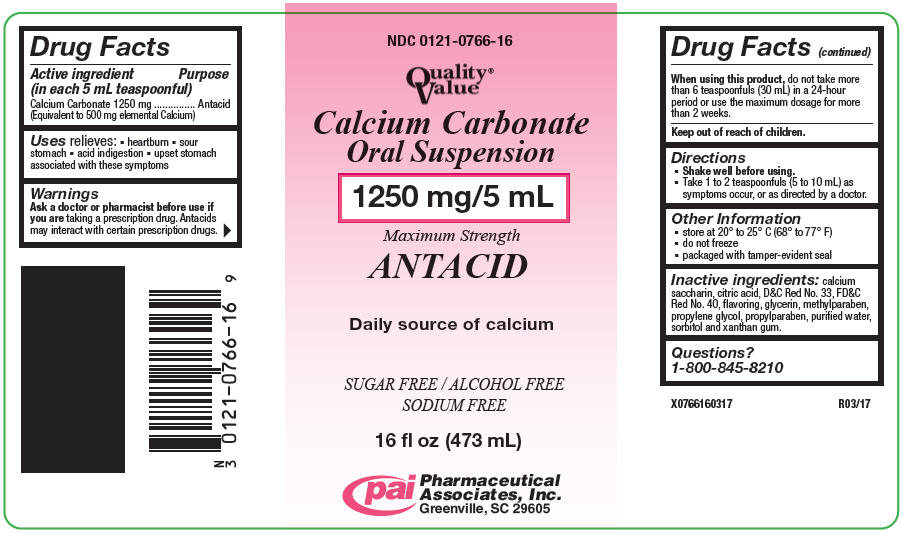
Active ingredient (in each 5 mL teaspoonful)
Calcium Carbonate 1250 mg
(Equivalent to 500 mg elemental Calcium)
Warnings
Ask a doctor or pharmacist before use if you are taking a prescription drug. Antacids may interact with certain prescription drugs.
Directions
- Shake well before using.
- Take 1 to 2 teaspoonfuls (5 to 10 mL) as symptoms occur, or as directed by a doctor.
Other information
- store at 20° - 25°C (68° - 77°F)
- do not freeze
- Calcium Carbonate Oral Suspension is a pink-colored, bubble gum flavored suspension supplied in the following oral dosage forms:
| NDC 0121-0766-16: | 16 fl oz (473 mL) bottle |
| NDC 0121-4766-05: | 5 mL unit dose cup, in a tray of ten cups. |
Inactive ingredients
calcium saccharin, citric acid, D&C Red No. 33, FD&C Red No. 40, flavoring, glycerin, methylparaben, propylene glycol, propylparaben, purified water, sorbitol and xanthan gum.
Questions or comments?
Call 1-800-845-8210.
You may also report serious side effects to this phone number.