Label: IBUPROFEN tablet, film coated
-
NDC Code(s):
43063-872-06,
43063-872-10,
43063-872-20,
43063-872-30, view more43063-872-40, 43063-872-82, 43063-872-90
- Packager: PD-Rx Pharmaceuticals, Inc.
- This is a repackaged label.
- Source NDC Code(s): 49483-602
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated September 27, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
- ibuprofen tablets 400 mg - 600 mg- 800 mg medguide
-
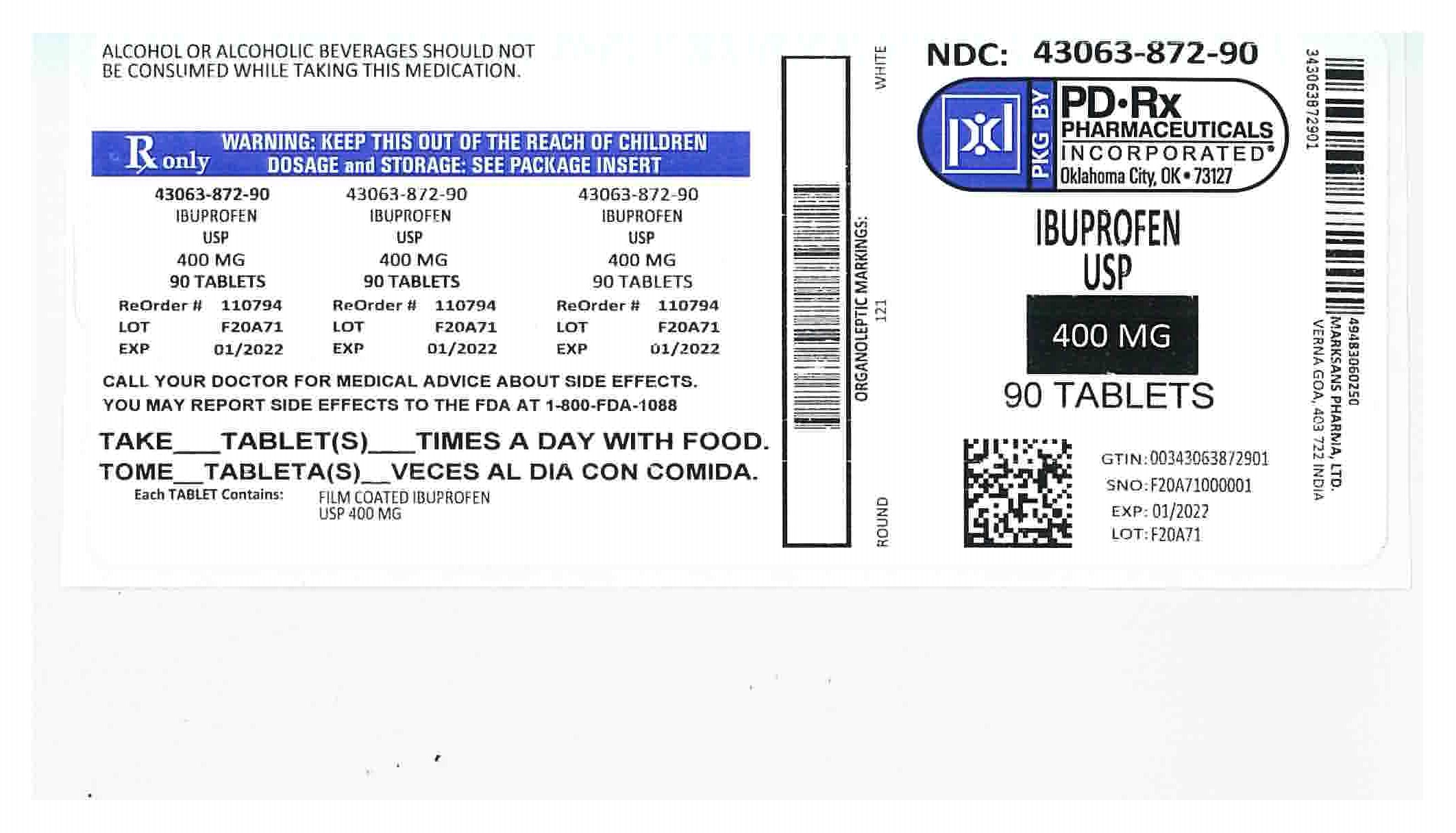
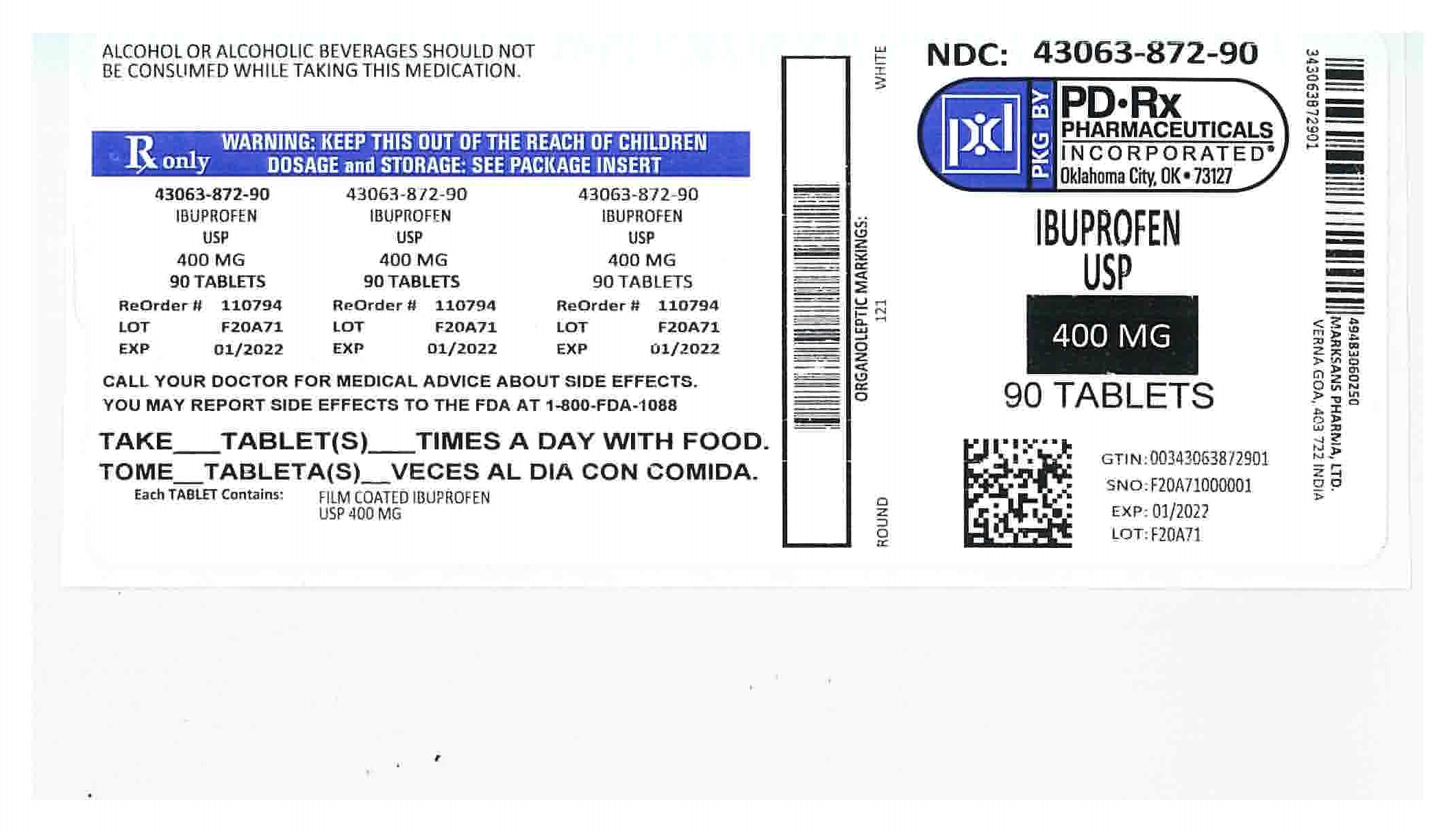
HOW SUPPLIED
400mg (white to of white, round, biconvex, film coated tablets debossed with '121' on one side and plain on the other side) Bottles of:
NDC 43063-872-06 Bottles of 6
NDC 43063-872-10 Bottles of 10
NDC 43063-872-20 Bottles of 20
NDC 43063-872-30 Bottles of 30
NDC 43063-872-40 Bottles of 40
NDC 43063-872-90 Bottles of 90
NDC 43063-872-82 Bottles of 500
- 400mg Ibuprofen label
-
INGREDIENTS AND APPEARANCE
IBUPROFEN
ibuprofen tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:43063-872(NDC:49483-602) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IBUPROFEN (UNII: WK2XYI10QM) (IBUPROFEN - UNII:WK2XYI10QM) IBUPROFEN 400 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POLYVINYL ALCOHOL (UNII: 532B59J990) STARCH, PREGELATINIZED CORN (UNII: O8232NY3SJ) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color white Score no score Shape ROUND Size 13mm Flavor Imprint Code 121 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:43063-872-06 6 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 01/25/2019 2 NDC:43063-872-10 10 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 04/29/2019 3 NDC:43063-872-20 20 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 08/07/2018 4 NDC:43063-872-30 30 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 08/07/2018 5 NDC:43063-872-40 40 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 08/07/2018 6 NDC:43063-872-90 90 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 04/26/2019 7 NDC:43063-872-82 500 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 03/02/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA090796 12/30/2015 Labeler - PD-Rx Pharmaceuticals, Inc. (156893695) Registrant - PD-Rx Pharmaceuticals, Inc. (156893695) Establishment Name Address ID/FEI Business Operations PD-Rx Pharmaceuticals, Inc. 156893695 repack(43063-872)