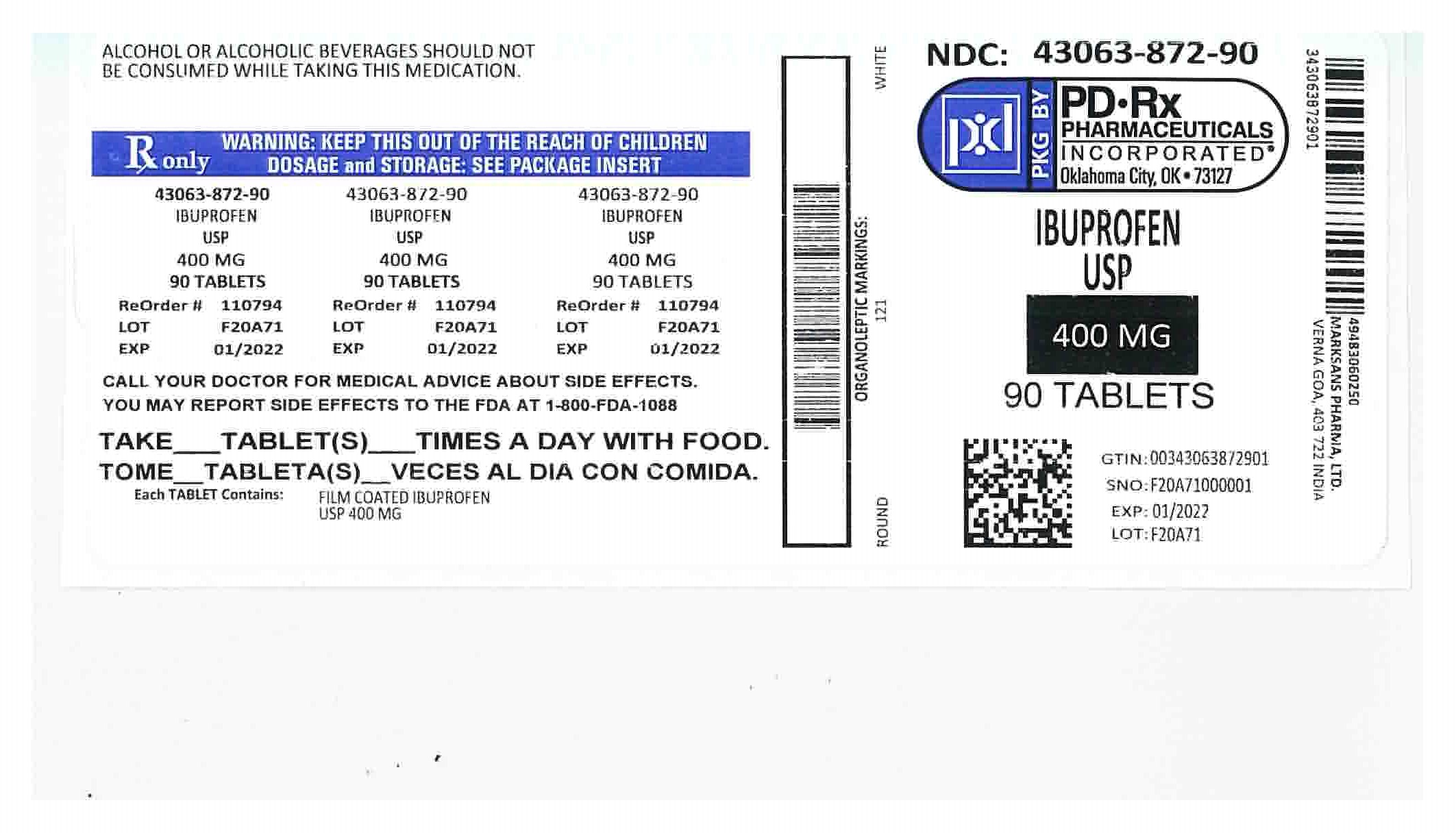
HOW SUPPLIED
400mg (white to of white, round, biconvex, film coated tablets debossed with '121' on one side and plain on the other side) Bottles of:
NDC 43063-872-06 Bottles of 6
NDC 43063-872-10 Bottles of 10
NDC 43063-872-20 Bottles of 20
NDC 43063-872-30 Bottles of 30
NDC 43063-872-40 Bottles of 40
NDC 43063-872-90 Bottles of 90
NDC 43063-872-82 Bottles of 500