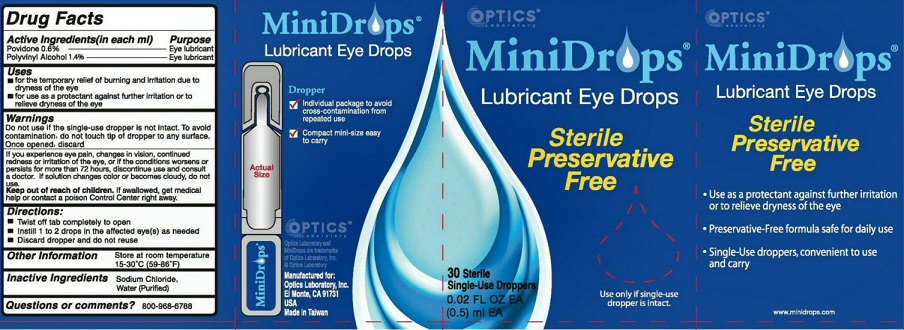
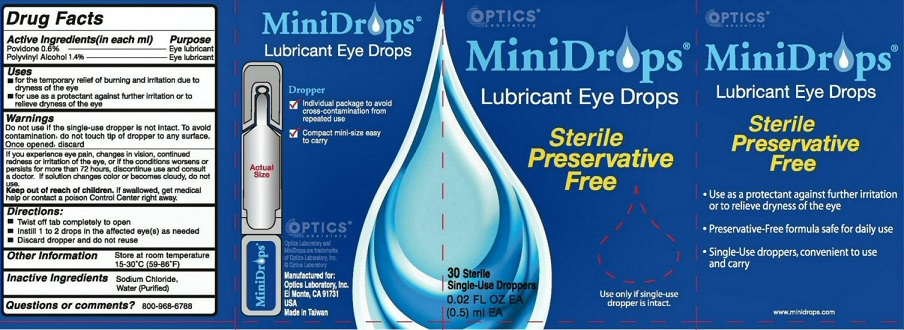
Label: MINIDROPS- lubricant eye drops liquid
- NDC Code(s): 64108-212-10, 64108-212-12, 64108-212-13, 64108-212-65
- Packager: Optics Laboratory, Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 16, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENTS SECTION
- PURPOSE SECTION
- KEEP OUT OF REACH OF CHILDREN SECTION
- INDICATIONS AND USE SECTION
-
WARNINGS SECTION
WARNINGS DO NOT USE IF THE SINGLE-DOSE DROPPER IS NOT INTACT. TO AVOID CONTAMINATION, DO NOT TOUCH TIP OF DROPPER TO ANY SURFACE. ONCE OPENED, DISCARD.
IF YOU EXPERIENCE EYE PAIN, CHANGES IN VISION, CONTINUED REDNESS OR IRRITATION OF THE EYE, OR IF THE CONDITIONS WORSENS OR PERSISTS FOR MORE THAN 72 HOURS, DISCONTINUE USE AND CONSULT A DOCTOR. IF SOLUTION CHANGES COLOR OR BECOMES CLOUDY, DO NOT USE
OTHER INFORMATION STORE AT ROOM TEMPERATURE 15-30 DEGREES C (59-86 DEGREES F)
- DOSAGE AND ADMINISTRATION SECTION
- INACTIVE INGREDIENTS SECTION
- QUESTIONS SECTION
-
PACKAGE LABEL
MINIDROPS LUBRICANT EYE DROPS DROPPER
INDIVIDUAL PACKAGE TO AVOID CROSS-CONTAMINATION FROM REPEATED USE
COMPACT MINI-SIZE EASY TO CARRY OPTICS MANUFACTURED FOR OPTICS LABORATORY INC.EL MONTE, CA 91731 USA MADE IN TAIWAN
STERILE PRESERVATIVE FREE USE ONLY IF SINGLE-USE DROPPER IS INTACT USE AS A PROTECTANT AGAINST FURTHER IRRITATION OR TO RELIEVE DRYNESS OF THE EYE PRESERVATIVE-FREE FORMULA SAFE FOR DAILY USE SINGLE-USE DROPPERS, CONVENIENT TO USE AND CARRY
MINIDROPS AND OPTIC LABORATORY ARE TRADEMARKS OF OPTIC LABORATORY INC


res
-
INGREDIENTS AND APPEARANCE
MINIDROPS
lubricant eye drops liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:64108-212 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) (POVIDONE - UNII:FZ989GH94E) POVIDONE, UNSPECIFIED 6.0 mg in 1 mL POLYVINYL ALCOHOL (UNII: 532B59J990) (POLYVINYL ALCOHOL - UNII:532B59J990) POLYVINYL ALCOHOL 14.0 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) .9 mg in 1 mL WATER (UNII: 059QF0KO0R) 97.1 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:64108-212-13 5 in 1 BOX 04/01/1991 1 NDC:64108-212-10 0.5 mL in 1 VIAL, SINGLE-USE; Type 0: Not a Combination Product 2 NDC:64108-212-12 30 in 1 BOX 04/01/1991 2 NDC:64108-212-10 0.5 mL in 1 VIAL, SINGLE-USE; Type 0: Not a Combination Product 3 NDC:64108-212-65 65 in 1 BOX 04/01/1991 3 NDC:64108-212-10 0.5 mL in 1 VIAL, SINGLE-USE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M018 04/01/1991 Labeler - Optics Laboratory, Inc (018503552) Registrant - Optics Laboratory, Inc (018503552) Establishment Name Address ID/FEI Business Operations Taiwan Biotech 656127933 manufacture(64108-212)