KEEP OUT OF REACH OF CHILDREN SECTION
KEEP OUT OF REACH OF CHILDREN. IF SWALLOWED, GET MEDICAL HELP OR CONTACT A POISON CONTROL CENTER RIGHT AWAY.
INDICATIONS AND USE SECTION
USES FOR TEMPORARY RELIEF OF BURNING AND IRRITATION DUE TO DRYNESS OF THE EYE
FOR USE AS A PROTECTANT AGAINST FURTHER IRRITATION OR TO RELIEVE DRYNESS OF THE EYE
WARNINGS SECTION
WARNINGS DO NOT USE IF THE SINGLE-DOSE DROPPER IS NOT INTACT. TO AVOID CONTAMINATION, DO NOT TOUCH TIP OF DROPPER TO ANY SURFACE. ONCE OPENED, DISCARD.
IF YOU EXPERIENCE EYE PAIN, CHANGES IN VISION, CONTINUED REDNESS OR IRRITATION OF THE EYE, OR IF THE CONDITIONS WORSENS OR PERSISTS FOR MORE THAN 72 HOURS, DISCONTINUE USE AND CONSULT A DOCTOR. IF SOLUTION CHANGES COLOR OR BECOMES CLOUDY, DO NOT USE
OTHER INFORMATION STORE AT ROOM TEMPERATURE 15-30 DEGREES C (59-86 DEGREES F)
DOSAGE AND ADMINISTRATION SECTION
DIRECTIONS TWIST OFF TAB COMPLETELY TO OPEN. INSTILL 1 TO 2 DROPS IN THE AFFECTED EYE(S) AS NEEDED. DISCARD DROPPER AND DO NOT REUSE.
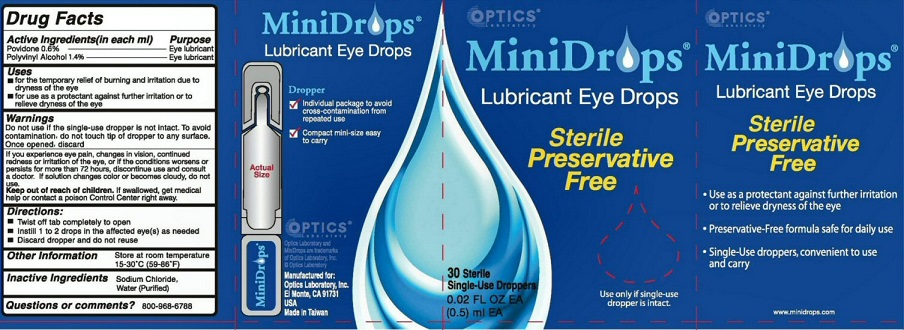
PACKAGE LABEL
MINIDROPS LUBRICANT EYE DROPS DROPPER
INDIVIDUAL PACKAGE TO AVOID CROSS-CONTAMINATION FROM REPEATED USE
COMPACT MINI-SIZE EASY TO CARRY OPTICS MANUFACTURED FOR OPTICS LABORATORY INC.EL MONTE, CA 91731 USA MADE IN TAIWAN
STERILE PRESERVATIVE FREE USE ONLY IF SINGLE-USE DROPPER IS INTACT USE AS A PROTECTANT AGAINST FURTHER IRRITATION OR TO RELIEVE DRYNESS OF THE EYE PRESERVATIVE-FREE FORMULA SAFE FOR DAILY USE SINGLE-USE DROPPERS, CONVENIENT TO USE AND CARRY
MINIDROPS AND OPTIC LABORATORY ARE TRADEMARKS OF OPTIC LABORATORY INC


res