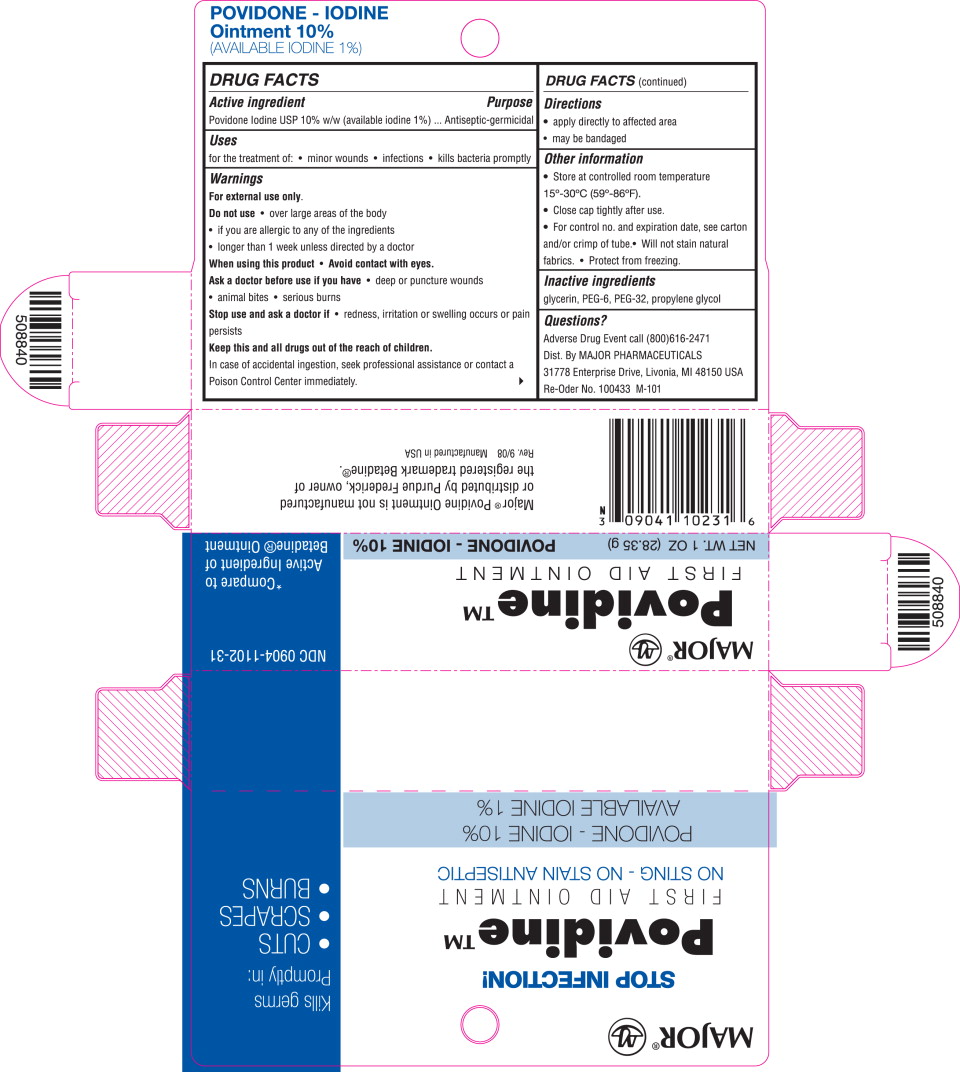
Label: MAJOR POVIDONE IODINE- povidone-iodine ointment
- NDC Code(s): 0904-1102-31
- Packager: Major Pharmaceuticals
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
- Warnings
- Directions
- Other information
- Inactive ingredients
- Questions?
- Principal Display Panel – 1 oz. Tube
- Principal Display Panel – 1 oz. Carton
-
INGREDIENTS AND APPEARANCE
MAJOR POVIDONE IODINE
povidone-iodine ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0904-1102 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POVIDONE-IODINE (UNII: 85H0HZU99M) (IODINE - UNII:9679TC07X4) IODINE 100 mg in 1 g Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) POLYETHYLENE GLYCOL 300 (UNII: 5655G9Y8AQ) POLYETHYLENE GLYCOL 1500 (UNII: 1212Z7S33A) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0904-1102-31 1 in 1 CARTON 08/14/2006 1 28.35 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M005 08/14/2006 Labeler - Major Pharmaceuticals (191427277)