MAJOR POVIDONE IODINE- povidone-iodine ointment
Major Pharmaceuticals
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
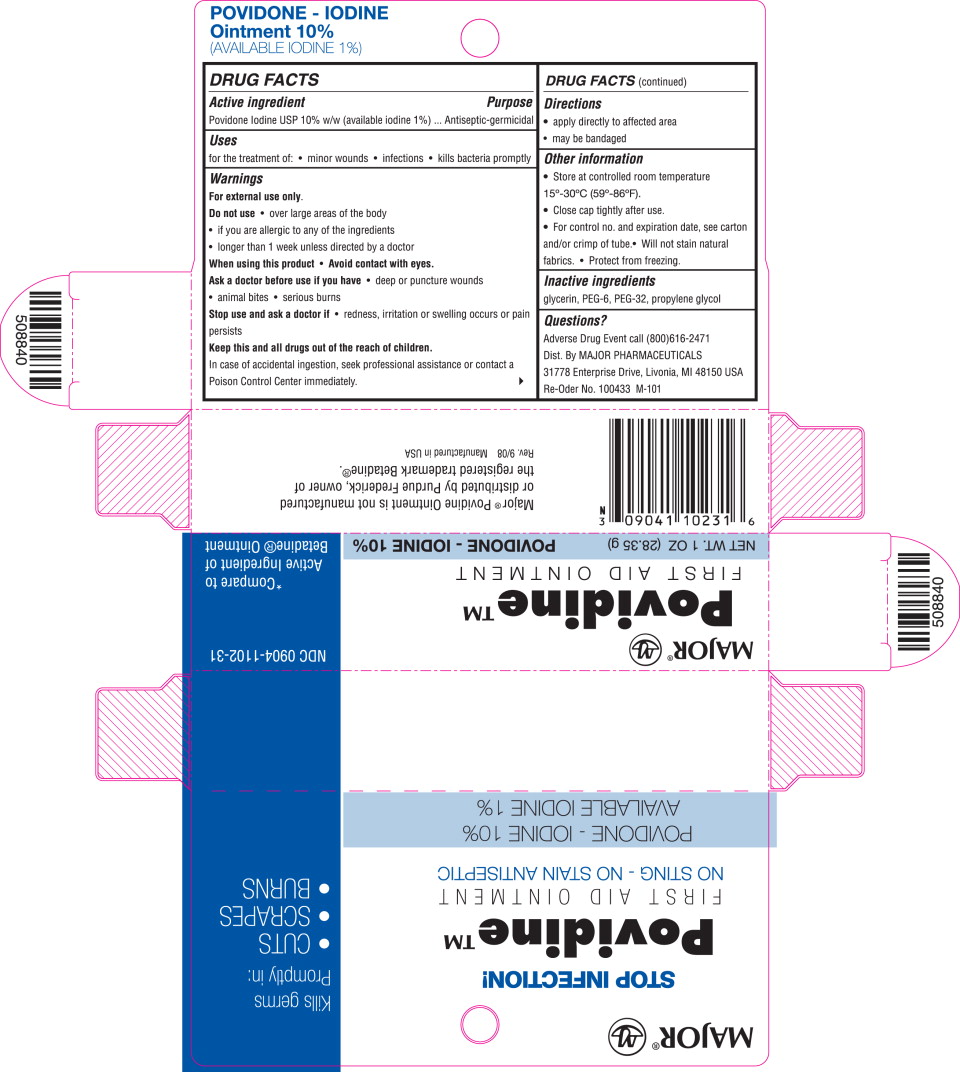
Active ingredient
Povidone Iodine USP10% w/w (available iodine 1%)
Purpose
Antiseptic-germicidal
Uses
for the treatment of:
- minor wounds
- infections
- kills bacteria promptly
Warnings
For external use only.
Do not use
- over large areas of the body
- if you are allergic to any of the ingredients
- longer than 1 week unless directed by a doctor
Ask a doctor before use if you have
- deep or puncture wounds
- animal bites
- serious burns
Stop use and ask a doctor if
- redness, irritation or swelling occurs or pain persists
Keep this and all drugs out of the reach of children.
In case of accidental ingestion, seek professional assistance or contact a Poison Control Center immediately.
Directions
- apply directly to affected area
- may be bandaged
Other information
- Store at controlled room temperature 15°-30°C (59°-86°F).
- Close cap tightly after use.
- For control no. and expiration date, see carton and/or crimp of tube.
- Will not stain natural fabrics.
- Protect from freezing.
Inactive ingredients
glycerin, PEG-6, PEG-32, propylene glycol
Questions?
Adverse Drug Event call (800)616-2471
Dist. By MAJOR PHARMACEUTICALS
31778 Enterprise Drive, Livonia, Ml 48150 USA
Re-Oder No. 100433 M-101
Principal Display Panel – 1 oz. Tube
Major® NDC 0904-1102-31
Povidine™
FIRST AID OINTMENT
NET WT. 1 OZ (28.35 g) *Compare to
POVIDINE – IODINE 10% Active Ingredient of
AVAILABLE IODINE 1% Betadine® Ointment

Principal Display Panel – 1 oz. Carton
Major® NDC 0904-1102-31
Povidine™ *Compare to
FIRST AID OINTMENT Active Ingredient of
NET WT. 1OZ (28.35 g) POVIDINE - IODINE 10% Betadine® Ointment