Label: OLOPATADINE HYDROCHLORIDE solution/ drops
- NDC Code(s): 62332-709-05
- Packager: Alembic Pharmaceuticals Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 17, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
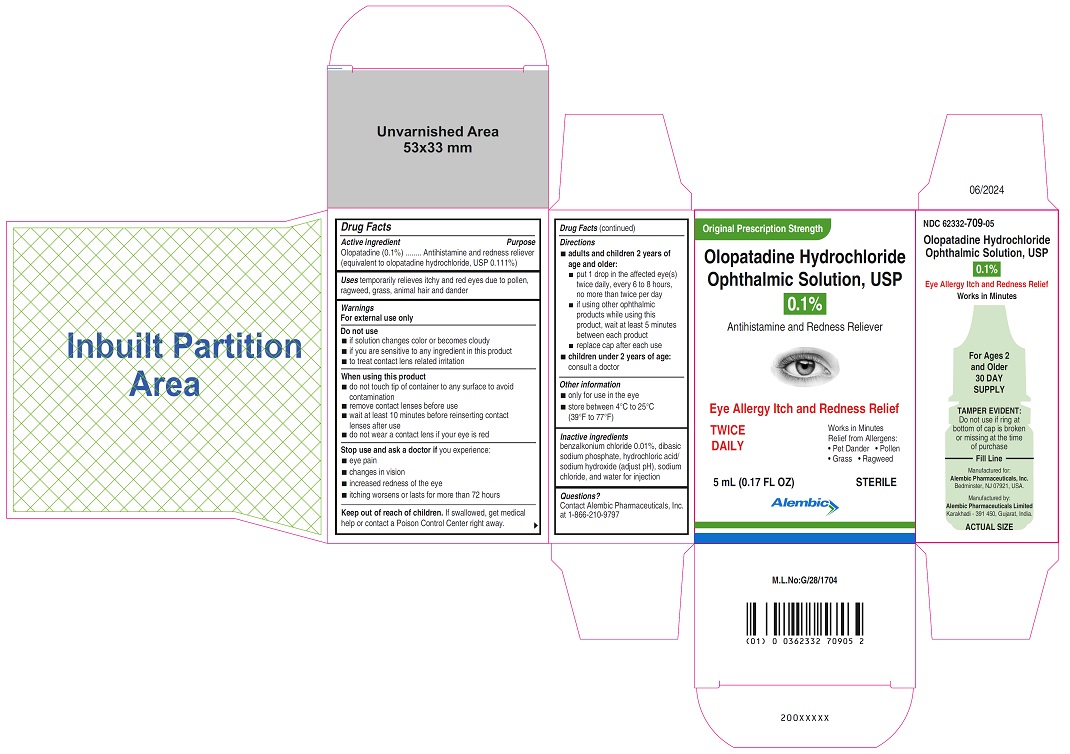
- ACTIVE INGREDIENT(S)
- PURPOSE
- USE(S)
- WARNINGS
- DO NOT USE
- WHEN USING THIS PRODUCT
- STOP USE AND ASK DOCTOR IF
- KEEP OUT OF REACH OF CHILDREN
-
DIRECTIONS
• adults and children 2 years of age and older:
• put 1 drop in the affected eye(s) twice daily, every 6 to 8 hours, no more than twice per day
• if using other ophthalmic products while using this product, wait at least 5 minutes between each product
• replace cap after each use
• children under 2 years of age: consult a doctor - OTHER INFORMATION
- INACTIVE INGREDIENTS
- QUESTIONS?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
OLOPATADINE HYDROCHLORIDE
olopatadine hydrochloride solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62332-709 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OLOPATADINE HYDROCHLORIDE (UNII: 2XG66W44KF) (OLOPATADINE - UNII:D27V6190PM) OLOPATADINE HYDROCHLORIDE 1 mg in 1 mL Inactive Ingredients Ingredient Name Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) SODIUM PHOSPHATE, DIBASIC (UNII: GR686LBA74) HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) SODIUM CHLORIDE (UNII: 451W47IQ8X) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62332-709-05 1 in 1 CARTON 02/18/2021 1 5 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA209919 02/18/2021 Labeler - Alembic Pharmaceuticals Inc. (079288842) Establishment Name Address ID/FEI Business Operations Gland Pharma Limited 918601238 MANUFACTURE(62332-709) Establishment Name Address ID/FEI Business Operations Alembic Pharmaceuticals Limited (F3) 675480734 MANUFACTURE(62332-709)