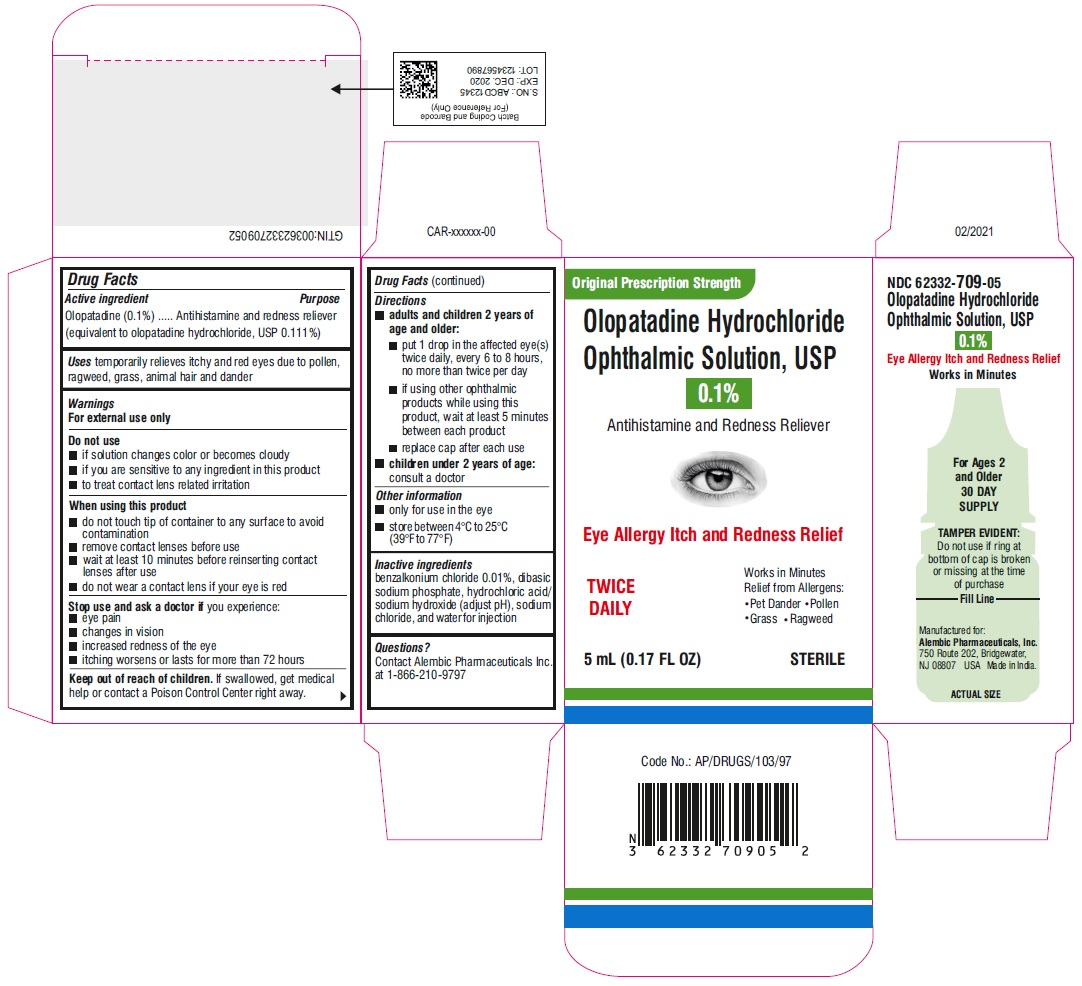
DO NOT USE
• if solution changes color or becomes cloudy
• if you are sensitive to any ingredient in this product
• to treat contact lens related irritation
WHEN USING THIS PRODUCT
• do not touch tip of container to any surface to avoid contamination
• remove contact lenses before use
• wait at least 10 minutes before reinserting contact lenses after use
• do not wear a contact lens if your eye is red
STOP USE AND ASK DOCTOR IF
you experience:
- eye pain
- changes in vision
- increased redness of the eye
- itching worsens or lasts for more than 72 hours
KEEP OUT OF REACH OF CHILDREN
If swallowed, get medical help or contact a Poison Control Center right away.
DIRECTIONS
• adults and children 2 years of age and older:
• put 1 drop in the affected eye(s) twice daily, every 6 to 8 hours, no more than twice per day
• if using other ophthalmic products while using this product, wait at least 5 minutes between each product
• replace cap after each use
• children under 2 years of age: consult a doctor