Label: WELL AT WALGREENS- sodium bicarbonate, sodium chloride powder, for solution
- NDC Code(s): 0363-0802-00
- Packager: Walgreen Company
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated December 18, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- PURPOSE
- INDICATIONS & USAGE
- KEEP OUT OF REACH OF CHILDREN
- WARNINGS
- DOSAGE & ADMINISTRATION
-
INSTRUCTIONS FOR USE
Instructions for use
USES:
Temporarily relieves symptoms associated with sinusitis, colds, flu or allergies, including nasal stuffiness, sneezing, runny nose and post-nasal
drip. Removes inhaled irritants (e.g. dust, pollen, smoke). Promotes nasal and sinus drainage. Helps reduce swelling of nasal membranes.
DOSAGE:
Adults and children age 4 years and older may use 1-2 packets of dry ingredients administered as described below as often as
every 2 hours. For children under age 4 consult a health care professional before use.
DIRECTIONS:
Read through entire section before using for the first time.
1. Remove the cap from the Walgreens Kettle Neti Pot.
2. Pour the pre-mixed saline dry into the Walgreens Kettle Neti Pot. First-time users
should start with one packet of the saline dry ingredients.As you become more accustomed to the system, work up to using 2 full packets.
Additional packets may be purchased from your nearest Walgreens pharmacy.
3. Fill the pot to the 8 oz line with distilled, sterile, or filtered water (using a filter with an absolute pore size of 1 micron or smaller)
or previously boiled (boil water 1 minute or 3 minutes if at elevations above 6,500 feet then allow to cool to body temperature) water.
This makes 1/2 cup (4 oz) of solution.
4. Tighten the cap on the pot securely, place one finger over the spout and one over the hole on the cap and gently shake the pot until
the dry ingredients have completely dissolved..
4. Proper head position allows the solution to run through the nose by gravity.
-Lean over the sink so you are looking directly into the basin. Holding the pot in your right hand, gently insert the spout
into your right nostril so that it forms a comfortable seal.
-Rotate your head so that the right nostril is directly above the left. The forehead should remain higher than the chin. Raise the handle of the pot so
that the solution enters the right nostril. In a few moments, the solution will begin to drain out of the left nostril into the sink. Do not inhale or "snort"
solution into the nose - breathe through your mouth.
6. When the pot is empty, exhale through both nostrils to clear them of excess mucous and solution. Gently blow your nose into a tissue.
7. Repeat the procedure on the other side.
7. Thoroughly rinse the pot after each use with water that has been distilled, sterilized, filtered or previously boiled and ;eave the pot to air dry completely.
The Walgreens Kettle Neti-Pot is top-rack dishwasher safe.
- ACTIVE INGREDIENT
- INACTIVE INGREDIENT
- OTHER SAFETY INFORMATION
- QUESTIONS
- OTHER SAFETY INFORMATION
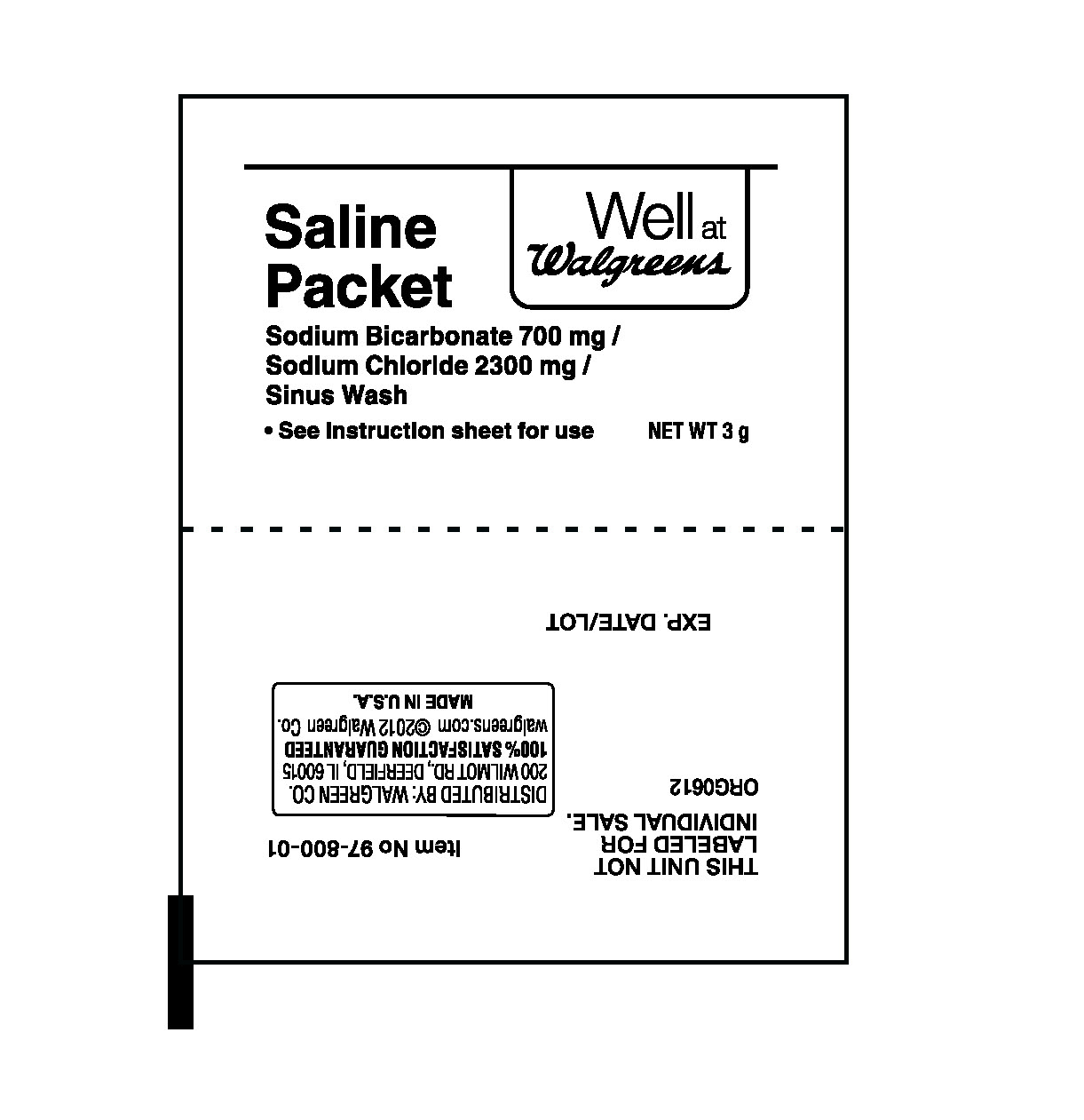
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
WELL AT WALGREENS
sodium bicarbonate, sodium chloride powder, for solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0363-0802 Route of Administration NASAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM BICARBONATE (UNII: 8MDF5V39QO) (SODIUM CATION - UNII:LYR4M0NH37, BICARBONATE ION - UNII:HN1ZRA3Q20) SODIUM BICARBONATE 700 mg in 3000 mg SODIUM CHLORIDE (UNII: 451W47IQ8X) (SODIUM CATION - UNII:LYR4M0NH37) SODIUM CHLORIDE 2300 mg in 3000 mg Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0363-0802-00 3000 mg in 1 PACKET; Type 0: Not a Combination Product 04/01/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 04/01/2013 Labeler - Walgreen Company (008965063)